Overall Score4
- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
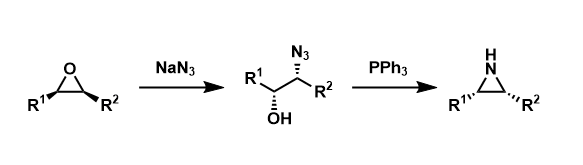
Epoxides can be converted into aziridines by nucleophilic attack of azide ion, the Staudinger reaction, and aziridine ring formation with elimination of triphenylphosphine oxide.
-
General References
- Ittah, Y.; Shahak, I.; Blum, J. J. Org. Chem. 1978, 43, 397. DOI: 10.1021/jo00397a004
- Ittah, Y.; Sasson, Y.; Shahak, I.; Tsaroom, S.; Blum. J. J. Org. Chem. 1978, 43, 4271. DOI: 10.1021/jo00416a003
-
Reaction Mechanism
The Staudinger azide reduction and elimination of PPh3=O leads to the formation of aziridines. Note that both C-N bond forming steps are stereospecific SN2 displacement.

-
Examples
-
Experimental Procedure
-
Experimental Tips
-
References
-
Related Reactions
-
Related Books
-
External Links
Aziridines in Synthesis (PDF, Baran’s group)