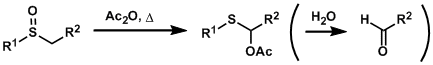- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
The treatment of sulfoxides with acid anhydrides or acid chlorides brings about a reaction called the Pummerer rearrangement, which gives α-acyloxythioethers.
The rearranged products can be hydrolyzed to give aldehydes. Therefore, sulfoxides and sulfides can be considered as aldehyde precursors.
-
General References
・Pummerer, R. Ber. 1909, 42, 2282.
・Pummerer, R. Ber. 1910, 43, 1401.
・De Lucchi, O. et al. Org. React. 1991, 40, 157.
・Grierson, D. S.; Husson, H.-P. Comp. Org. Syn. 1991, 6, 924.
・Padwa, A.; Gunn, D. E., Jr.; Osterhout, M. H. Synthesis 1997, 1353. DOI: 10.1055/s-1997-1384
・Padwa, A.; Rur, S. K.; Danca, M. d.; Ginn, J. D.; Lynch, S. M. Synlett 2002, 851. DOI: 10.1055/s-2002-31891
・Bur, S. K.; Padwa, A. Chem. Rev. 2004, 104, 2401. DOI: 10.1021/cr020090l
-
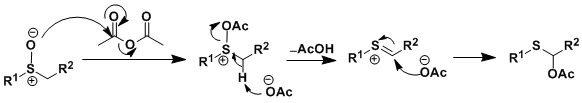
Reaction Mechanism
Ref: Chem. Commun. 2006, 2786.
-
Examples
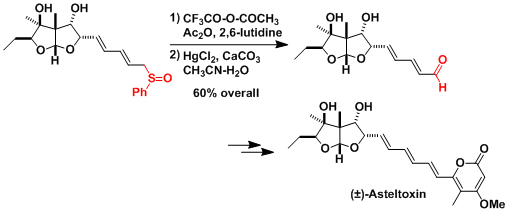
The total synthesis of asteltoxin.[1]
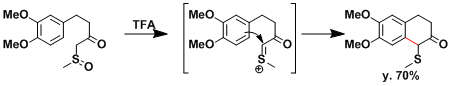
An example where the rearrangement is applied to effect intramolecular C-C bond formation.
The total synthesis of ent-hyperforin.[2]
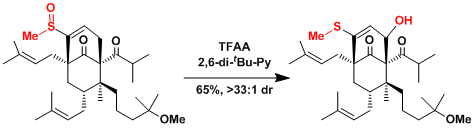
-
Experimental Procedure
-
Experimental Tips
-
References
[1] Schreiber, S. L.; Satake, K. J. Am. Chem. Soc. 1984, 106, 4186. DOI: 10.1021/ja00327a020
[2] Shimizu, Y.; Shi, S-L.; Usuda, H.; Kanai, M.; Shibasaki, M. Angew. Chem., Int. Ed. 2010, 49, 1103. doi:10.1002/anie.200906678
-
Related Books
[amazonjs asin=”0198558996″ locale=”US” title=”Organosulfur Chemistry (Oxford Chemistry Primers)”]
[amazonjs asin=”3662147122″ locale=”US” title=”Organosulfur Chemistry II (Topics in Current Chemistry) (Volume 205)”]