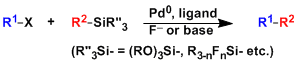- Generality
- Reagent Availability
- Experimental User Friendliness
- Criteria #4
- Criteria #5
-
General Characteristics
The cross coupling reaction between aryl halides/triflates and organosilicon reagents (organosilanes) is generally called the Hiyama coupling.
Most organosilicon reagents are highly stable and the coupling reaction utilizes Lewis basic activators such as fluorides to generate transmetallation-active hypervalent silicate species.
The silicon atom usually has to contain heteroatom or aryl substituent(s). Trialkylsilyl reagents are more difficult to be converted into reactive silicates and thus less suited for the cross coupling.
This reaction has a high potential considering the low toxicity and environmental impact of organosilanes. However, the synthesis of the necessary heteroatom-containing organosilanes is still underdeveloped and the reaction is consequently not used as widely as the alternative reactions such as the Suzuki-Miyaura coupling.
Vinylsilanes can be synthesized by alkyne hydrosilylation. In this case, it is possible to obtain the regioselectivities that are inaccessible by hydroboration.
-
General References
・Hatanaka, Y.; Hiyama, T. J. Org. Chem. 1988, 53, 918. doi:10.1021/jo00239a056
・Hiyama, T.; Hatanaka, Y. Pure. Appl. Chem. 1994. 66, 1471.
・Hiyama, T. J. Organomet. Chem. 2002, 653, 58.
・Denmark, S. E.; Obar, M. H. Aldrichimica Acta 2003, 36, 75. [PDF]
-
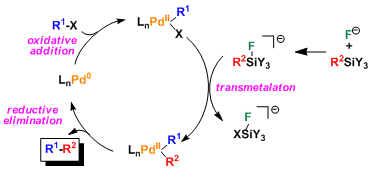
Reaction Mechanism
Stable organosilanes need to be activated first to silicate species by Lewis bases such as fluorides.
-
Examples
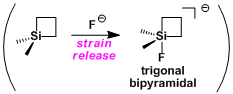
Silacyclobutanes have unusually high Lewis acidity resulting from the ring strain. They can be used in the Hiyama coupling under mild conditions.[1] 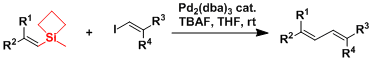
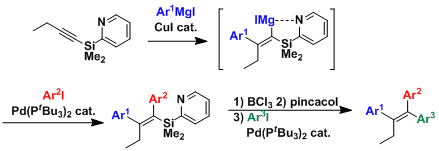
The cross coupling using the chelating pyridyl silyl group allows for the preparation of difficult tetrasubstituted olefins with perfect geometric selectivity. [2]
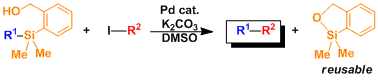
The specially designed phenylsilane derivative allows the coupling reaction to take place without fluorides. The siloxane byproduct can be recovered and recycled.[3]
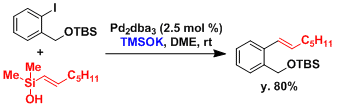
Organic silanols can be used in the Hiyama coupling using inexpensive TMSOK as the Lewis base.[4] This allows for the use of substrates containing functionalities unstable against fluorides.
-
Experimental Procedure
-
Experimental Tips
-
References
[1] Denmark, S. E.; Choi, J. Y. J. Am. Chem. Soc. 1999, 121, 5821. DOI: 10.1021/ja9908117
[2] Itami, K.; Kamei, T.; Yoshida,J.-i. J. Am. Chem. Soc. 2003, 125, 14670. DOI: 10.1021/ja037566i
[3] Nakao, Y.; Imanaka, H.; Sahoo, A. H.; Yada, A.; Hiyama, T. J. Am. Chem. Soc. 2005, 127, 6952. DOI: 10.1021/ja051281j
[4] (a) Denmark, S. E.; Sweis, R. F. J. Am. Chem. Soc. 2001, 123, 6439. DOI: 10.1021/ja016021q For mechanistic implications, see: (b) Denmark, S. E. et al. J. Am. Chem. Soc. 2004, 126, 4865. DOI: 10.1021/ja037234d (c) Denmark, S. E.; Sweis, R. F. J. Am. Chem. Soc. 2004, 126, 4876. DOI: 10.1021/ja0372356
-
Related Books
[amazonjs asin=”3527305181″ locale=”US” title=”Metal-Catalyzed Cross-Coupling Reactions (2 Volume Set)”]
[amazonjs asin=”3527319379″ locale=”US” title=”Modern Arylation Methods”]
[amazonjs asin=”0470850337″ locale=”US” title=”Palladium Reagents and Catalysts: New Perspectives for the 21st Century”]
[amazonjs asin=”364206308X” locale=”US” title=”Palladium in Organic Synthesis (Topics in Organometallic Chemistry)”]
[amazonjs asin=”3540421750″ locale=”US” title=”Cross-Coupling Reactions: A Practical Guide (Topics in Current Chemistry)”]