Seki, Y.; Tanabe, K.; Sasaki, D.; Sohma, Y.; Oisaki, K.; Kanai, M. Angew. Chem. Int. Ed. 2014, 53, Early View.
DOI: 10.1002/anie.201402618
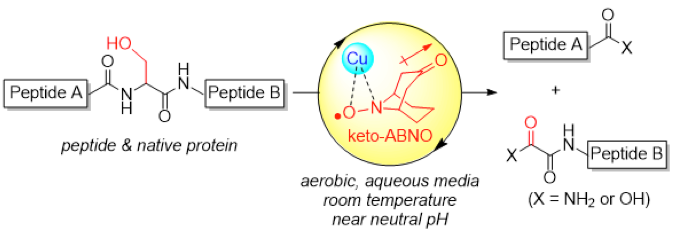
The site-specific cleavage of peptide bonds is an important chemical modification of biologically relevant macromolecules. The reaction is not only used for routine structural determination of peptides, but is also a potential artificial modulator of protein function. Realizing the substrate scope beyond the conventional chemical or enzymatic cleavage of peptide bonds is, however, a formidable challenge. Here we report a serine-selective peptide-cleavage protocol that proceeds at room temperature and near neutral pH value, through mild aerobic oxidation promoted by a water-soluble copper–organoradical conjugate. The method is applicable to the site-selective cleavage of polypeptides that possess various functional groups. Peptides comprising d-amino acids or sensitive disulfide pairs are competent substrates. The system is extendable to the site-selective cleavage of a native protein, ubiquitin, which comprises more than 70 amino acid residues.
Site-selective cleavage of peptides by chemical reaction which shows beyond enzymatic scope is important technology for structural determination and potential functional modulation of proteins.
Big challenges are mildness of the conditions and chemoselectivity. Peptide (amide) bond is one of strongest chemical bonds consists of biomolecules, but cleavage reactions should be conductible under near physiological conditions and a number of sensitive functional groups present in peptides and proteins should be tolerated.
Oisaki and Kanai group developed a novel oxidative serine(Ser)-selective peptide cleavage conductible at room temperature and near neutral pH. The key of success is combinational use of water-soluble copper catalyst, sterically less-hindered and electron-deficient organoradical (keto-ABNO[1]) developed by themselvesm, and a NOx source.
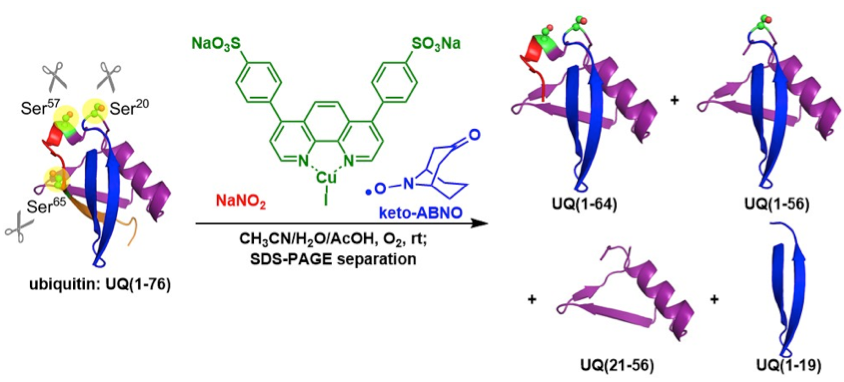
This system was applicable even to peptides possessing sensitive disulfide bonds and unnatural amino acids, which are out of scope of peptidase digestion. The conditions also cleaved native protein, ubiquitin (76 amino acids reasidues) with high fidelty. The chain cleavage predominantly occurred at three serine residues (Ser20, Ser57, Ser65) based on the analysis of SDS-PAGE and MALDI-TOF-MS analysis.
-
Reference
[1] “Catalytic Aerobic Production of Imines en Route to Mild, Green, and Concise Derivatizations of Amines”
Sonobe, T.; Oisaki, K.; Kanai, M. Chem. Sci. 2012, 3, 3249. DOI: 10.1039/C2SC20699D
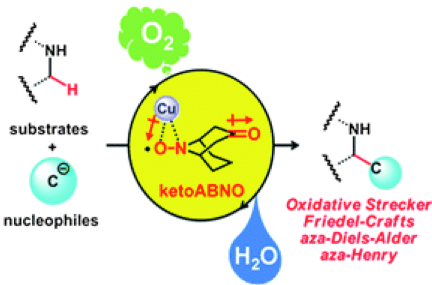
The development of a general, mild, and chemoselective catalytic aerobic oxidation of amines toimines is described. The combination of a less sterically demanding and electron-deficient new N-oxyl radical (ketoABNO: 5) and copper(I) salt is key for the high catalytic activity and allows for the use of molecular oxygen as the stoichiometric oxidant producing H2O as the sole side-product. The novel method is extendable to a direct α-derivatization of secondary amines via sequential aerobic oxidation of amines to imines followed by C–C bond-formation to the resulting imines, including the novel catalytic asymmetric aerobic cross-dehydrogenative coupling reaction. Mechanistic insight into the novel catalytic system is also discussed.
-
Related Book
[amazonjs asin=”3527318674″ locale=”US” title=”Peptides: Chemistry and Biology”]
-
Related Link
Kanai Laboratory