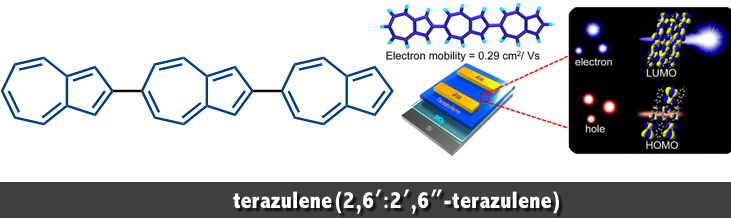
2,6′ :2′ ,6″ -terazulene, wherein three azulene units are connected linearly in the same direction. This molecule has the potential to enhance the unique properties of the azulene unit, including the internal dipole moment and the diff erent distribution of HOMO and LUMO, derived from the nonalternant hydrocarbon character. In 2013, the first synthesis and characterization of 2,6′ :2′ ,6″ -terazulene has been achieved by Nakayama and Katagiri at Yamagata University in Japan.[1]
-
References
Yamaguchi, Y.; Ogawa, K.; Nakayama, K.-I.; Ohba, Y.; Katagiri, H. J. Am. Chem. Soc. 2013, 135, 19095–19098. DOI: 10.1021/ja410696j
We present herein a linear expanded π-conjugation system comprising azulene units:2,6′:2′,6″-terazulene. This simple hydrocarbon exhibits excellent n-type transistor performance with an electron mobility of up to 0.29 cm2 V–1 s–1. The lowest unoccupied molecular orbital (LUMO) is well distributed over the entire molecule, whereas the highest occupied molecular orbital (HOMO) is localized at one end. These findings indicate a disadvantage of hole carrier transport and an advantage of n-type-specific transport behavior. This system presents an unconventional concept: polarity control of OFET by molecular orbital distribution control.
-
Related Books
[amazonjs asin=”352732934X” locale=”US” title=”Aromaticity and Other Conjugation Effects”]