Hadlington, T. J.; Hermann, M.; Frenking, G.; Jones, C. J. Am. Chem. Soc. 136, 2014. 136, 3028.
DOI: 10.1021/ja5006477
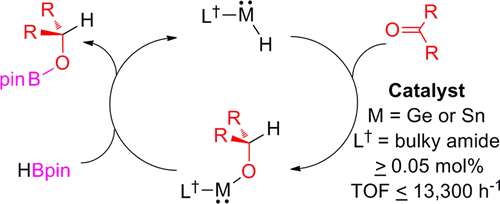
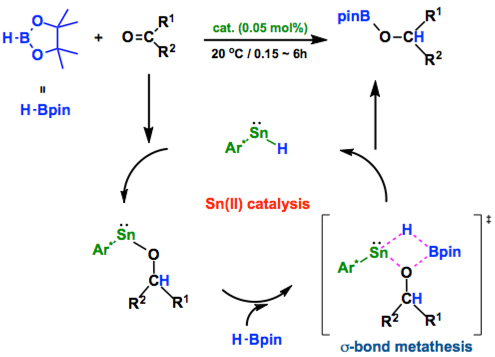
This study details the first use of well-defined low-valent p-block metal hydrides as catalysts in organic synthesis. That is, the bulky, two-coordinate germanium(II) and tin(II) hydride complexes, L†(H)M: (M = Ge or Sn, L† = −N(Ar†)(SiiPr3), Ar† = C6H2{C-(H)Ph2}2iPr-2,6,4), are shown to act as efficient catalysts for the hydroboration (with HBpin, pin = pinacolato) of a variety of unactivated, and sometimes very bulky, carbonyl compounds. Catalyst loadings as low as 0.05 mol % are required to achieve quantitative conversions, with turnover frequencies in excess of 13 300 h−1 in some cases. This activity rivals that of currently available catalysts used for such reactions.
As everyone knows nowadays about the Frustrated Lewis Pair (FLP) chemistry, the recent development of main-group catalysis is remarkable. Here, new entry of the catalytically active p-block compounds has just appeared. Jones group revealed that both Ge(II) and Sn(II) hydride species is pretty efficient for the catalytic hydroboration of ketones. Note that this is the first example for the catalysis involving low-valent p-block metal although there are few reports main group metal with the normal oxidation state such as Mg(II) before.[1]
Addition of Sn-H or Ge-H bond to C=O followed by the s-bond metathesis between E-O (E = Sn, Ge) and H-Bpin completes the catalytic cycle. Because the reaction mechanism is pretty similar to the case with the Mg catalyst,[1] all reactions succeeded with the Mg complex will work with the Sn(II) or Ge(II) compounds described here too. In fact, Jones mentions that this protocol can apply for other reactions such as CO2 reduction and alkene hydrosilylations.
-
References
[1] “Magnesium-catalyzed hydroboration of aldehydes and ketones”
Arrowsmith, M.; Hadlington, T. J.; Hill, M. S.; Kociok-Kőhn. G. Chem. Commun. 2012, 48, 4567 – 4569. DOI:10.1039/c2cc30565h
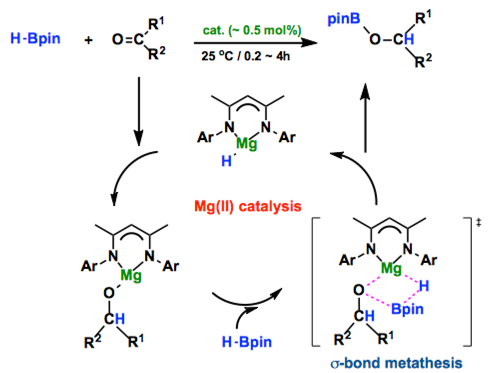
The heteroleptic magnesium alkyl complex [CH{C(Me)NAr}2-MgnBu] (Ar = 2,6-iPr2C6H3) is reported as a highly efficient pre-catalyst for the hydroboration of aldehydes and ketones with pinacol borane.
-
Related Books
[amazonjs asin=”3642377580″ locale=”US” title=”Frustrated Lewis Pairs II: Expanding the Scope (Topics in Current Chemistry)”][amazonjs asin=”B00EZ1CF6C” locale=”US” title=”Frustrated Lewis Pairs II. Expanding the Scope”][amazonjs asin=”B00EZ1S17O” locale=”US” title=”Frustrated Lewis Pairs I. Uncovering and Understanding”]
-
Related Links
Jones Lab home Page
Frenking Lab home Page
Hill Lab home Page