Carbon-carbon bond formation is a basic reaction in organic chemistry, which builds the carbon skeleton in organic molecules. A vast number of carbon-carbon bond-forming reactions have been developed up to now. Nonetheless, development of new bond forming reactions enables innovative synthetic routes towards known and novel compounds, along with reducing the number of steps towards complex molecules.
When developing new bond forming reactions, it is important to take into account the functional groups attached to the carbon backbone. Functional groups are usually reactive, but if the bond forming reaction takes place without disrupting the functionalities, the reaction would be applicable towards the synthesis of complex natural products. Functional group tolerance is therefore an important factor to consider when developing organic reactions.
Last year in 2014, a new catalytic olefin coupling reaction was reported by the group of Prof. Phil S. Baran at the Scripps Institute in the United States.
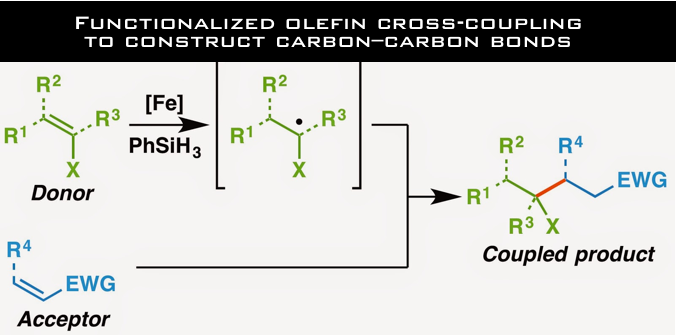
“Functionalized olefin cross-coupling to construct carbon–carbon bonds”
Lo, J. C.; Gui, L.; Yabe, Y.; Pan, C.–M.; Baran, P. S. Nature 2014, 516, 343. DOI:10.1038/nature14006
This reaction is not only a new carbon-carbon bond-forming reaction, but exhibits remarkable functional group tolerance.
Reductive coupling reaction of olefins by an iron catalyst
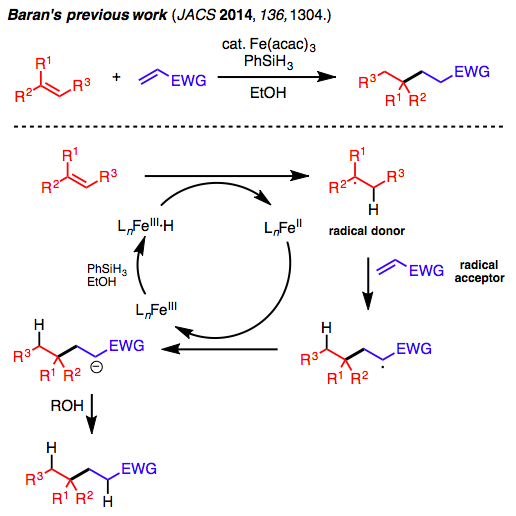
The iron catalyzed reductive cross-coupling reaction that forms carbon-carbon bonds between different olefins has been developed based on a previous report by Baran’s group at the beginning of 2014.[1]
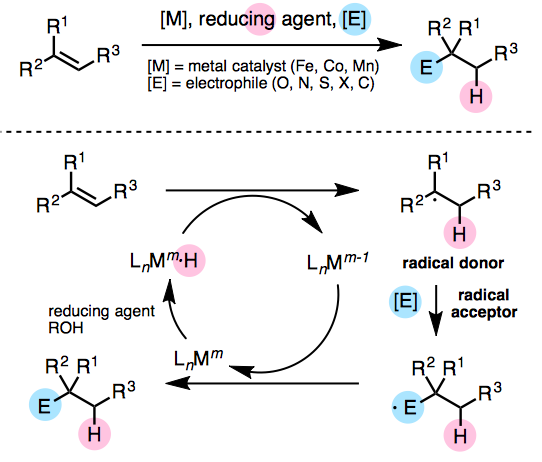
Hydrofunctionalization of olefins is a well known reductive coupling reaction of olefins. The hydroboration of olefins developed by Herbert C. Brown, where hydrogen and boron are attached to the double bond is an example of a hydrofunctionalization. In recent years, catalytic hydrofunctionalizations where transition metals such as iron, cobalt and manganese have been developed, where the reactions proceed in the presence of relatively non-expensive metal catalysts under mild conditions.[2]
Catalytic hydrofunctionalizations are considered to proceed via a radical mechanism involving a one-electron redox reaction (Figure 1). The radical donor arising from the metal hydride complex couples with the electrophile (shown in blue) to generate the product.
By using olefins as the electrophile, it is possible to achieve reductive cross-coupling between olefins.
However, there have been no examples of carbon-carbon bond formation from the hydrofunctionalization of olefins, and the coupling between different olefins, i.e. reductive cross-coupling has been an unexplored area.
Baran and his co-workers investigated the reaction system with an iron catalyst and developed a system where tertiary or secondary radicals (donors) couple with olefins bearing electron-withdrawing groups (acceptors) as shown in Figure 2. The reaction conditions were optimized based on hydrofunctionalization of olefins using Fe2(ox)2·6H2O and NaBH4, which was developed by the group of Prof. Dale L. Boger, who is also from the Scripps Institute.[3] Baran’s group has developed a reaction system using Fe(acac)3/PhSiH3, which possess high catalytic activity.
Cross-coupling of functionalized olefins
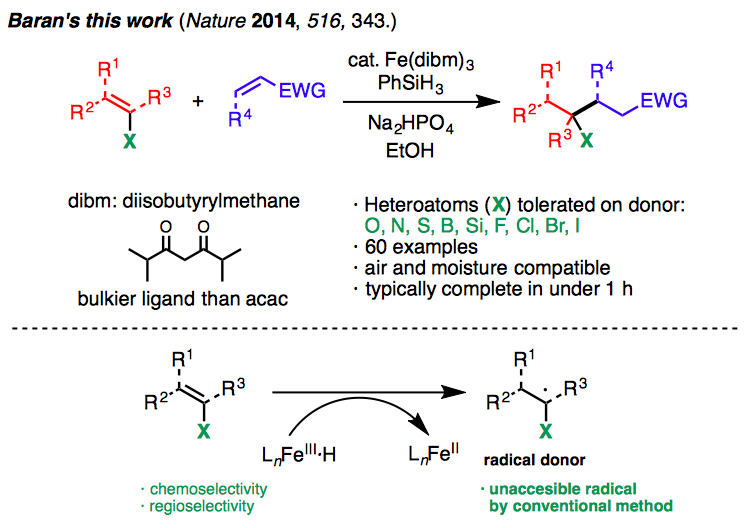
Baran’s research that was published in Nature, demonstrates the successful coupling of olefins that possess various functionalities such as boronic acids, esters, thioethers and halogens. Although functional groups are important anchors for subsequent bond formations, they often interfere with the desired reactions.
Even in this reaction, the electronic properties of some functional groups lead to difficulty in controlling the regioselectivity of the arising radicals. In addition, the need to control possible oxidative addition and/or transmetallation with the metal catalyst, along with elimination of functional groups makes the coupling reaction extremely challenging.
Therefore, Baran’s group used Fe(dibm)3 in the place of Fe(acac)3, to control the regioselectivity of radical generation and suppress side reactions, whilst achieving the coupling of functionalized olefins. Fe(dibm)3 has a lower Lewis acidity compare to Fe(acac)3, and is thus effective for the reactions with olefins having Lewis basic functionalities.
It is noteworthy that over 60 olefins, which have functionalities containing oxygen, nitrogen, sulfur, boron, silicon and halogen have been demonstrated to be applicable for this cross-coupling reaction. Although there is still room for improvement in the reaction yields and further tuning of reaction conditions are required, it is remarkable that such a challenging reaction could be performed with a wide range of substrates.
In addition, it should be noted that this method allows the generation of radicals from compounds arising from haloalkyls, which has been difficult according to previous methods. Other advantages of this method are that the reactions can be performed in air and are typically complete within 1 hour.
Transformation of molecules whilst keeping highly reactive functional groups intact is a common challenge encountered in the total synthesis of compounds that involve numerous steps. Therefore, the installation of functional groups without interfering with other functionalities under mild conditions is extremely useful for the construction of carbon skeletons. Although the molecules synthesized in this research have a simple structure, over 90% of the compounds are reported to be new compounds. This suggests the possibility of this method to synthesize other novel and useful compounds.
Baran’s group has their own blog, where they introduce hints of their reaction and share the difficulties encountered in their synthesis. A movie clip is also provided for this cross-coupling reaction.
Through tuning of ligands and reaction conditions of this cross-coupling reaction, it is expected that the list of applicable substrates will be extended and that this reaction will be effective for synthesizing useful compounds.
References
[1] Lo, J. C.; Yabe, Y.; Pan, C.-M.; Baran, P. S. J. Am. Chem. Soc. 2014, 136, 1304. DOI:10.1021/ja4117632 [2] Isayama, S.; Mukaiyama, T. Chem. Lett. 1989, 18, 1071. DOI: 10.1246/cl.1989.1071 [3] Ishikawa, H.; Colby, D. A.; Boger, D. L. 2008, 130, 420. DOI: 10.1021/ja078192m
Japanese: http://www.chem-station.com/blog/2015/01/olefincoupling.html