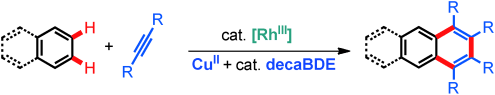Pham, M. V.; Cramer, N. Angew. Chem. Int. Ed. 2014. Early View.
DOI: 10.1002/anie.201310723
Larger condensed arenes are of interest owing to their electro- and photochemical properties. An efficient synthesis is the catalyzed aromatic annulation of a smaller arene with two alkyne molecules. Besides difunctionalized starting materials, directed C–H functionalization can be used for such aromatic homologation. However, thus far the requirement of either pre-functionalized substrates or suitable directing groups were limiting this approach. Herein, we describe a rhodium(III)-catalyzed method allowing the use of completely unbiased arenes and internal alkynes. The reaction works best with copper(II) 2-ethylhexanoate and decabromo- diphenyl ether as the oxidant combination. This aromatic annulation tolerates a variety of functional groups and delivers homologated condensed arenes. Aside from simple benzenes, naphthalenes and higher condensed arenes provide access to highly substituted and highly soluble acenes structures having important electronic and photophysical properties.
π-extention of aromatic compounds leading larger condensed arenes and heteroarenes have received significant attention by chemists. A modular and attractive method for their synthesis is an arene homologation that provides the next higher acene by the condensation of an aromatic ring with two alkyne units.
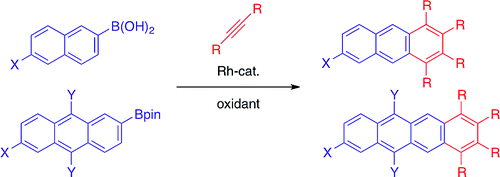
In 2001, Sato and Miura, Osaka Universitry, has been reported the Rh-catalyzed annulation of arylboronic acids with internal alkynes which can be activated at single C–H bond.[1] On the other hand, in this paper, Creamer[2] who is working in Laboratory of Asymmetric Catalysis and Synthesis, EPFL SB ISIC at Switzerland reported acene annulation by an undirected double oxidative Rh catalyzed C–H functionalization.
-
References
[1] “Synthesis of Highly Substituted Acenes through Rhodium-Catalyzed Oxidative Coupling of Arylboron Reagents with Alkynes”
Fukutani, T.; Hirano, K.; Satoh, T.; Miura, M. J. Org. Chem. 2011, 76, 2867. DOI: 10.1021/jo200339w
[2] See his recent representative work;
“Chiral Cyclopentadienyl Ligands as Stereocontrolling Element in Asymmetric C–H Functionalization”
Ye B.: Cramer, N. Science 2012, 338, 504.
Metal complexes coordinated by a single cyclopentadienyl (Cp) ligand are widely used, versatile catalysts, but their application to asymmetric reactions has been hindered by the difficulty of designing Cp substituents that effectively bias the coordination sphere. Here, we report on a class of simple C2-symmetric Cp derivatives that finely control the spatial arrangement of the transiently coordinated reactants around the central metal atom. Rhodium(III) complexes bearing these ligands proved to be highly enantioselective catalysts for directed carbon-hydrogen (C–H) bond functionalizations of hydroxamic acid derivatives.
-
Related Books
[amazonjs asin=”1849735700″ locale=”US” title=”C-H and C-X Bond Functionalization: Transition Metal Mediation (RSC Catalysis Series)”]
-
Related Links
Cramer group