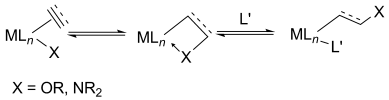Peng, X.; Tong, M. K. B.; Hirao,H.; Chiba, S. Angew. Chem. Int. Ed. 53, 2014. 1959.
DOI: 10.1002/anie.201308617
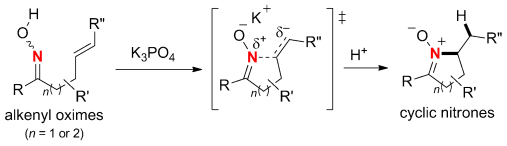
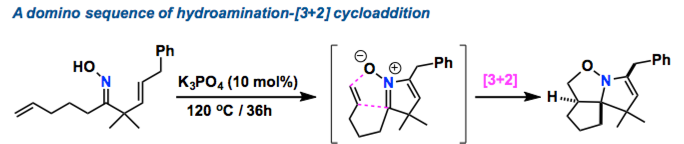
A method based on hydroamination mediated by inorganic base was developed for the synthesis of cyclic nitrones from alkenyl oximes. DFT calculations of the reaction pathway suggested that this hydroamination could proceed through an unprecedented nucleophilic amination of the unactivated alkene by the oxime nitrogen atom. The transition state of this reaction is stabilized by an ionic interaction between a metal cation such as K+ and the oxime oxygen and negatively charged alkene moiety.
Nitrogen-containing derivatives are very important molecule skeleton as found in natural products, agrochemicals, cosmetics, and pharmaceuticals. They are also efficient synthetic intermediates in various industrial processes. Never-the-less, there are the limitations in C–N bond formation with traditional methods. Therefore, a variety of homogeneous catalysts have been used to achieve the so-called hydroamination reaction, addition of N–H bonds over unsaturated C–E bonds.[1]Chiba group demonstrated that inorganic bases such as K3PO4 are effective for an intramolecular hydroamination of alkenyl oximes, which produced cyclic nitrones. By using chiral bases, this approach will be extended to the synthesis for asymmetric variants, Chiba mentions.
-
References
[1] “Migratory Insertion of Alkenes into Metal–Oxygen and Metal–Nitrogen Bonds”
Hanley, P. S.; Hartwig. J. F. Angew. Chem. Int. Ed. 53, 2013. 8510. DOI:10.1002/anie.201300134
The insertion of an unsaturated ligand into a MC or MH bond proceeds through migratory insertion, a fundamental organometallic reaction. Recent literature documents evidence of the migratory insertion of alkenes into an MO and MN bonds for alkene alkoxylation and alkene amination reactions, respectively. Herein we provide an overview of the literature and a perspective on how these recent experiments relate to classic experiments on CO and CN bond formation with alkene complexes of the late transition metals.
-
Related Books
[amazonjs asin=”0471744980″ locale=”US” title=”Nitrile Oxides, Nitrones and Nitronates in Organic Synthesis: Novel Strategies in Synthesis (Organic Nitro Chemistry)”]
-
Related Links
Chiba Lab home Page