Braunschweig, H.; Dewhurst, R. D.; Hcrl, C.; Phukan, A. K.; Pinzner, F.; Ullrich, S. Angew. Chem. Int. Ed. 53, 2014. Early View.
DOI: 10.1002/anie.201309325
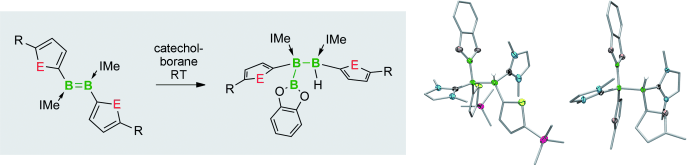
Synthetic access to electron-precise boron chains is hampered by the preferential formation of nonclassical structures. The few existing strategies for this involve either strongly reducing reagents or transition-metal catalysts, both with distinct disadvantages. The synthesis of new furyl- and thienyl-substituted diborenes is presented, along with their direct hydroboration with catecholborane (CatBH) to form a new electron-precise B=B bond and a B3 chain. The reaction is diastereoselective and proceeds under mild conditions without the use of strong reducing agents or transition-metal catalysts commonly used in B-B coupling reactions.
Braunschweig group is the top runner in the field of boron chemistry. They have shown unflagging energy in their pioneering research activity.
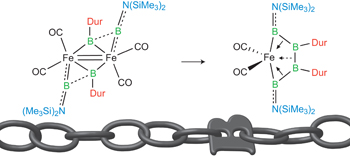
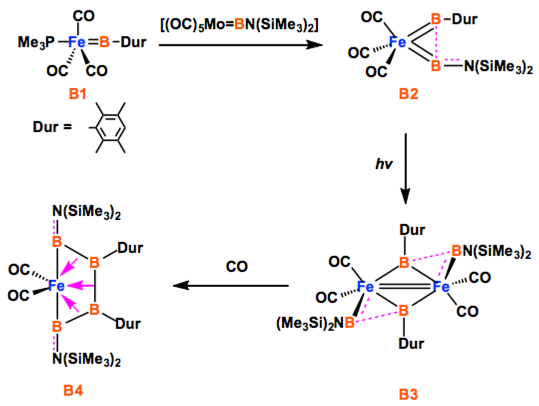
The way to make a boron chain -(B)n– by conventional synthesis is limited. Recently, Braunschweig group reported a homocatenation of boron on iron, which rendered a complex featuring a B4 chain.[1] In the title paper, they show a new methodology for the boron chain formation, thus direct hydroboration of B=B. The diastereoselective hydroboration proceeded at ambient temperature without catalysts, strong reductants, or any additives, and rendered bis(NHC)-stabilized triboranes B3. Braunschweig et al. will envisage the potential of the reaction to make longer B chains.
-
References
[1] “Controlled homocatenation of boron on a transition metal”
Braunschweig, H.; Ye, Q.; Vargas, A.; Dewhurst, R. D.; Radacki, K.; Damme, A. Nat. Chem. 2012, 4, 563. DOI: 10.1038/nchem.1379
Only a handful of elements are able to be controllably homocatenated (that is, to be formed into one- or two-dimensional chains or rings of the element), because most have weak element–element bonds. Boron forms strong B–B bonds, but its favourable cluster formation makes homocatenation very difficult. Recently, the coupling of borylene (:BR) ligands on a metal was predicted computationally. We have brought this prediction to fruition experimentally, and extended it by adding two further borylene units, stepwise forming a B4 chain bound to a metal under mild conditions. This complex is a useful model for understanding the metal–boron interactions required to promote transition of the boron atoms from borylene ligands to oligoborane networks bound side-on. The concept shows great promise for the controlled construction of one-dimensional boron chains.
-
Related Books
[amazonjs asin=”3540786333″ locale=”US” title=”Contemporary Metal Boron Chemistry I: Borylenes, Boryls, Borane Sigma-Complexes, and Borohydrides (Structure and Bonding)”]
-
Related Links
Braunschweig Lab home Page