McCarthy, S. M.; Lin, Y.-C.;Devarajan, D.;Chang, J. W.;Yennawar, H. P; Rioux, R. M.;Ess, D. H.;Radosevich, A. T. J. Am. Chem. Soc. 136, 2014. 136, 4640−4650
DOI: 10.1021/ja412469e
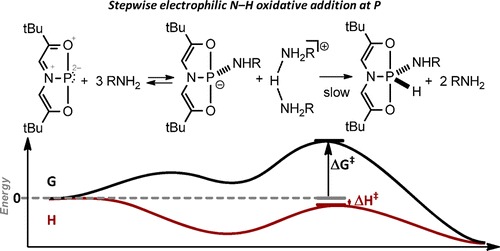
Ammonia, alkyl amines, and aryl amines are found to undergo rapid intermolecular N−H oxidative addition to a planar mononuclear σ3-phosphorus compound [P]. The pentacoordinate phosphorane products (P·[H][NHR]) are structurally robust, permitting full characterization by multinuclear NMR spectroscopy and single-crystal X-ray diffraction. Isothermal titration calorimetry was employed to quantify the enthalpy of the N−H oxidative addition of n-propylamine to [P]. The kinetics of n-propylamine N−H oxidative addition were monitored by in situ UV absorption spectroscopy and determination of the rate law showed an unusually large molecularity. Kinetic experiments conducted over the temperature range of 10−70 °C revealed that the reaction rate decreased with increasing temperature. Activation parameters extracted from an Eyring analysis (ΔH⧧= −0.8 ± 0.4 kcal/mol, ΔS⧧= −72 ± 2 cal/(mol·K)) indicate that the cleavage of strong N−H bonds by [P]is entropy controlled due to a highly ordered, high molecularity transition state. Density functional calculations indicate that a stepwise heterolytic pathway is preferred, proceeding by initial amine-assisted N−H heterolysis upon complexation to the electrophilic phosphorus center followed by rate controlling N → P proton transfer.
Historically, transition metal complexes have played a central role in the activation of strong bonds such as H-H, N-H, and C-H bonds in small molecule. That’s why they are utilized as efficient catalysts in synthetic chemistry. Even using the transition metal complexes, however, the activation of ammonia and using it as a substrate in catalysis is still regarded as a challenging. One of the reasons is that; as NH3 strongly bounds to metal center providing the stable “Werner complex”, it frequently kills the catalytic activity of metals due to the irreversible property under the mild conditions.
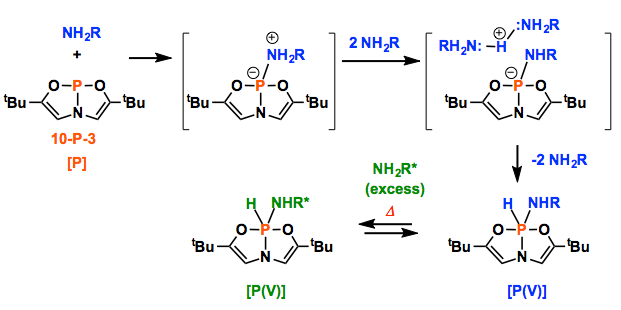
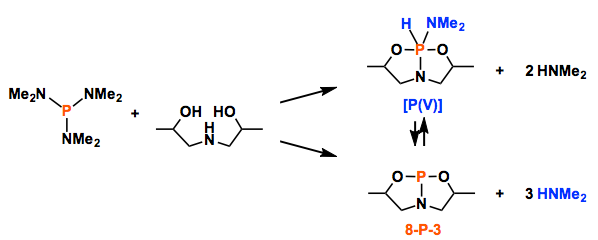
Here, Radosevich group demonstrates that 10-P-3 [P] activates the N-H bond of ammonia and amines under ambient temperature. The P(V) compounds were obtained via the oxidative addition of the N-H bond. In fact, such reaction was already reported in 1977~.[1] Important point here is that they could manage to reveal the molecular structure of the product. Although thermal reductive elimination experiments with the solid product ended up the failure, remarkably, the presence of excess amine led to amine exchange, which implies the reversible process of oxidative addition/reductive elimination as commonly found in transition metal catalysis. They also gained the mechanistic insight where two additional amines supported the proton transfer from N to P after the nucleophilic addition of N to P.
-
References
[1] For example, see
(a) “Sur Deux Phosphanes Bycycliques”
Houalla, D.; Osman, F .H.; Sanchez, M.; Wolf, R. Tetrahedron Lett.1977, 18, 3041. DOI: 10.1016/ S0040-4039(01)83152-X
(b) “Organo Phosphorus Tautomers Involving Change of Coordination Number on Phosphorus”
Wolf, R. Pure Appl. Chem.1980, 52, 1141. DOI: 10.1351/pac198052041141
(c) “The Chemistry of Organophosphorus Compounds” Burgada, R.; Setton, R., Ed by Hartley,F. Wiley: Chichester, 1994; 3, 185 − 272. ISBN: 0-471-93057-1
-
Related Books
[amazonjs asin=”B00BLQYKGM” locale=”US” title=”Transition Metal Organometallic Chemistry (SpringerBriefs in Molecular Science)”]
-
Related Links
Radosevich Lab home Page