Kahlert, J.; Stammler, H.-G.;Neumann, B.; Harder, R. A.; Weber, L.; Fox, M. A. Angew. Chem. Int. Ed. 53, 2014. Early View.
DOI: 10.1002/anie201310718
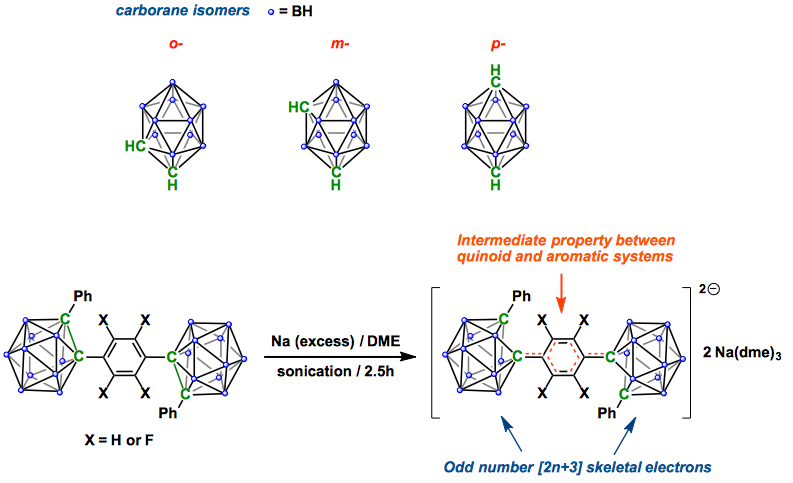
While carboranes with 2n+2 and 2n+4 (n= number of skeletal atoms) skeletal electrons (SE) are widelyknown, little has been reported on carboranes with odd SEnumbers. Electrochemical measurements on two-cage assemblies,where two C-phenyl-ortho-carboranyl groups are linkedby a para-phenylene or a para-tetrafluorophenylene bridge,revealed two well separated and reversible two-electronreduction waves indicating formation of stable dianions andtetraanions. The salts of the dianions were isolated by reductionwith sodium metal and their unusual structures were determinedby X-ray crystallography. The diamagnetic dianionscontain two 2n+3 SE clusters where each cluster has a notablylong carborane C–carborane C distance of ca 2.4 Å. The pconjugation within the phenylene bridge plays an importantrole in the stabilization of these carboranes with odd SE counts.
Icosahedral dicarba-closo-dodecaboranes, so what we call “carboranes”, have long attracted interest of synthetic researchers. Because of the unique three-dimensional structure, they are pretty stable thermally as well as chemically, and show unique properties such as electron delocalization within the s-framework. As shown below, three isomers are available depending on the position of carbon atoms in carborane and the functionalization or substituents on the C atoms are relatively easy, which has enriched the chemistry of this cluster.[1]
Here Weber and Fox groups report the reduction of the compounds featuring two carboranes linked at the para-position of benzene. The dianionic products involved the clusters with odd number [2n+3] skeletal electrons, and they revealed the molecular structures by X-ray diffraction. Interestingly, the central benzene linker showed an intermediate property between quinoid and aromatic systems; thus, two clusters communicate each other electronically through the p-conjugation system of benzene ring. By extending this method, it would be intriguing to see fluorescence properties of C-aryl carboranes, Weber and Fox imply.
-
References
[1] “Carbaboranes as Pharmacophores: Properties, Synthesis, and Application Strategies”
Scholz, M; Hey-Hawkins, E. Chem. Rev. 111, 2011.7035 – 7062. DOI: 10.1021/cr200038x
Geometry and electronic structure make carbaboranes unique artificial pharmacophores that are inert to catabolism, because corresponding enzymes are not known. Most frequently, carbaboranes are used as hydrophobic pharmacophores to address hydrophobic binding sites of biological targets. The icosahedral cluster with its very versatile chemistry allows attachment of all organic substituents that are necessary in drug design in mostly high yielding syntheses. The carbaboranyl – phenyl arrangement in general turned out to be a very good scaffold to address different targets by addition of particular substituents.
-
Related Links
Fox Lab home Page
Weber Lab home Page

![Crystal Structures of the Carborane Dianions [1,4-(PhCB10H10C)2C6H4]2- and [1,4-(PhCB10H10C)2C6F4]2- and the Stabilizing Role of the para-Phenylene Unit on 2n+3 Skeletal Electron Clusters](https://assets.en.chem-station.com/uploads/2014/04/for-toc.gif)