Wang, X.; Guo, P.; Han, Z.; Wang, X.; Wang, Z.; Ding, K. J. Am. Chem. Soc. 2014, 136, 405–411.
DOI: 10.1021/ja410707q
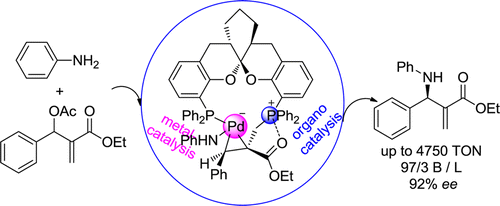
Exceptionally high activity (with a TON up to 4750) of the palladium complexes of SKP ligand was discovered in the catalysis of asymmetric allylic amination of MBH adducts with aromatic amines. A comprehensive mechanistic study indicates that the unique structural features of the SKP ligand, with a long P···P distance in its solid-state structure, were favorable for allowing two P atoms to play a bifunctional role in the catalysis. Herein, one of the P atom forms a C–P σ-bond with the terminal carbon atom of allyl moiety as a Lewis base, and an alternative P atom coordinates to Pd atom. The cooperative action of organo- and organometallic catalysis discovered in the present catalytic system is most likely responsible for its high activity, as well as excellent regio- and enantioselectivities. The mechanism disclosed in the present catalytic system is distinct from most of the currently recognized mechanisms for Pd-catalyzed allylic substitutions.

Ding Kuling, professor at Shanghai Institute of Organic Chemistry (SIOC) have recently developed a type of chiral spiroketal-based diphosphine ligands (SKP).[1] The palladium bearing their ligands were effective for asymmetric allylic amination of racemic Morita−Baylis−Hillman (MBH) adducts[2]
Morita-Baylis-Hillman Reaction
In this paper, they reported their new results on the discovery of exceptionally high activity of the catalysis, a long-standing challenge in this type of reaction, and the disclosure of a new mechanism based on the bifunctional role of SKP ligand.
-
References
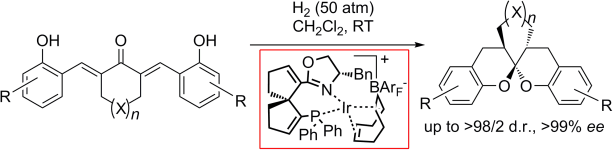
[1] “Catalytic Asymmetric Synthesis of Aromatic Spiroketals by SpinPhox/ Iridium(I)-Catalyzed Hydrogenation and Spiroketalization of a,a’- Bis(2-hydroxyarylidene) Ketones”
Wang, X.; Han, Z.; Wang, Z.; Ding, K. Angew. Chem. Int. Ed. 2012, 51, 936–940. DOI: 10.1002/anie.201106488
From spiro to spiro: An iridium(I) complex with a spiral P,N ligand (SpinPhox) is highly efficient in the catalytic asymmetric hydrogenation of α,α′-bis(2-hydroxyarylidene) ketones to afford the corresponding aromatic spiroketals in high yields with excellent diastereo- and enantioselectivities (see scheme). The complex plays a dual role in the reaction, acting as catalyst for both the hydrogenation of C![[DOUBLE BOND]](http://onlinelibrarystatic.wiley.com/undisplayable_characters/00f8fe.gif) C bonds and the subsequent spiroketalization of bisphenolic ketones.
C bonds and the subsequent spiroketalization of bisphenolic ketones.
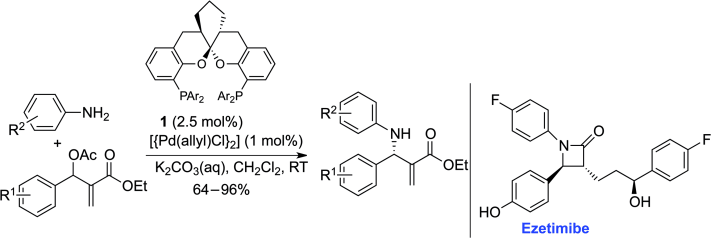
[2] “Aromatic Spiroketal Bisphosphine Ligands: Palladium-CatalyzedAsymmetric Allylic Amination of Racemic Morita–Baylis–HillmanAdducts”
Wang, X.; Meng, F.; Wang, Y.; Han, Z.; Chen, Y.-J.; Liu, L.; Wang, Z.; Ding, K. Angew. Chem. Int. Ed. 2012, 51, 9276–9282. DOI: 10.1002/anie.201204925
Showing a backbone: The spiroketal backbone of the bis(phosphine) ligand 1 led to good regio- and enantioselectivity in the palladium-catalyzed asymmetric allylic amination of racemic Morita–Baylis–Hillman adducts with aromatic amines. The methodology provides a facile and efficient synthesis of precursors for optically active β-lactam derivatives, including the cholesterol drug Ezetimibe.
- Related Links
Ding, Kuiling
-
Related Books
[amazonjs asin=”3527324895″ locale=”US” title=”Asymmetric Catalysis on Industrial Scale: Challenges, Approaches and Solutions”][amazonjs asin=”1891389548″ locale=”US” title=”Fundamentals Of Asymmetric Catalysis”]