Jat, J. L.; Paudyal, M. P.; Gao, H.; Xu, Q. L.; Yousufuddin, M.; Devarajan, D.; Ess, D. H.; Kurti, L.; Falck, J. R. Science 2014, 343, 61–65.
DOI: 10.1126/science.1245727
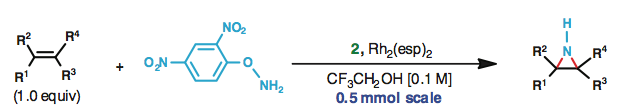
Despite the prevalence of the N-H aziridine motif in bioactive natural products and the clear advantages of this unprotected parent structure over N-protected derivatives as a synthetic building block, no practical methods have emerged for direct synthesis of this compound class from unfunctionalized olefins. Here, we present a mild, versatile method for the direct stereospecific conversion of structurally diverse mono-, di-, tri-, and tetrasubstituted olefins to N-H aziridines using O-(2,4-dinitrophenyl)hydroxylamine (DPH) via homogeneous rhodium catalysis with no external oxidants. This method is operationally simple (i.e., one-pot), scalable, and fast at ambient temperature, furnishing N-H aziridines in good-to-excellent yields. Likewise, N-alkyl aziridines are prepared from N-alkylated DPH derivatives. Quantum-mechanical calculations suggest a plausible Rh-nitrene pathway.
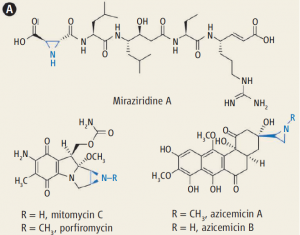
Aziridines, the triangular, comparably highly strained nitrogen analogs of epoxides, are important synthetic intermediates (i.e., building blocks) en route to structurally complex molecules because of their versatility in myriad regio- and stereoselective transformations (ring openings and expansions, as well as rearrangements).[1] The aziridine structural motif, predominantly N-H and to a lesser extent N-alkyl, also appears in biologically active natural products (e.g., azinomycins and mitomycins) as shown below.
Examples of biologically active, aziridine-containing natural products (DOI:10.1126/science.1248166)
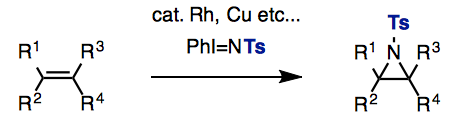
One method widely used for the synthesis of aziridines is the addition of nitrenes to olefins. Along these lines, sulfonamides serve as convenient nitrene precursors, which can be convertedusing commercially available iodobenzene diacetate in the presence of various transition-metal catalysts. Normally, the result is an aziridine bearing a strongly electron-withdrawing N-protecting group (e.g., Ts: para-toluenesulfonyl; Ns: para-nitrophenylsulfonyl); removal of these N-sulfonyl protecting groups is problematic as it often results in the undesired opening of the aziridine ring.
Falck and coworkers have achieved the development of method for the direct stereospecific conversion of olefins to N-H aziridines using O-(2,4-dinitrophenyl)hydroxylamine (DPH) via homogeneous rhodium catalysis with no external oxidants.
- References
[1] (a) Chawla, R.; Singh, A. K.; Yadav, L. D. S. RSC Adv. 2013, 3, 11385. DOI: 10.1039/c3ra00175j (b) Sweeney, J. Eur J. Org. Chem. 2009, 2009, 4911–4919. DOI: 10.1002/ejoc.200900211
- Related Links
Falck Lab Home Page
- Related Books
[amazonjs asin=”3527312137″ locale=”US” title=”Aziridines and Epoxides in Organic Synthesis”][amazonjs asin=”B003NHSR6G” locale=”US” title=”Aziridine: Webster’s Timeline History, 1951 – 2007″][amazonjs asin=”1175180270″ locale=”US” title=”Chemistry of aziridines”]