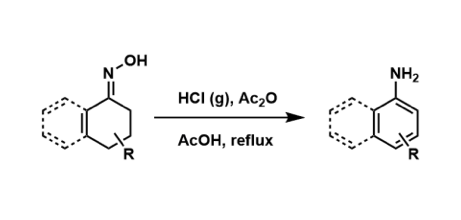
General Characteristics The synthesis of anilines from the corresponding cyclohexenone oximes is known as the Semmler-Wolff reaction. Due to the competing Beckmann rearrangement, yields tend to be ...
Posts by Category: Reactions
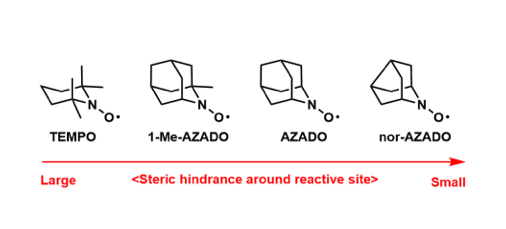
Nitroxyl Radical Oxidation Catalysts
General Characteristics Alkylated hydroxylamines are easily oxidized by oxygen in the air. When there is a proton on the carbon α to the nitrogen, hydroxylamines are oxidized to nitrones. However, ...
Vanadyl(IV) acetylacetonate
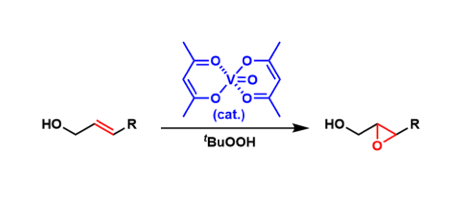
General Characteristics Vanadyl acetylacetonate (VO(acac)2) is used as a catalyst for epoxidation of allylic alcohols (with the use of tert-butyl hydroperoxide as the re-oxidant). The directing ...
Shenvi Isonitrile Synthesis
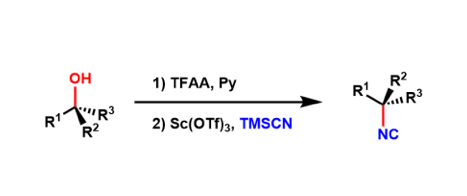
General Characteristics Shenvi reported recently that isonitriles can be prepared by substitution of tertiary alcohols using TMSCN with the inversion of stereochemistry. Isonitriles can be regarded ...
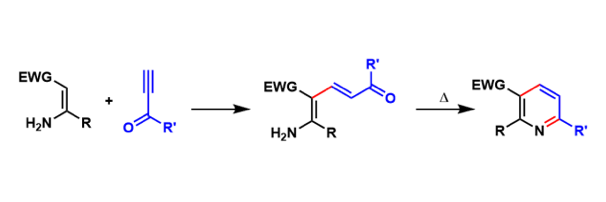
Bohlmann-Rahtz Pyridine Synthesis
General Characteristics Pyridines can be synthesized by condensation of appropriately substituted enamines and ethynyl ketones, which proceeds via aminodiene intermediates. General References ...
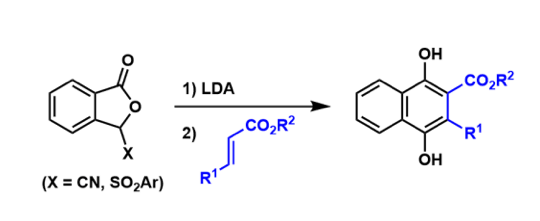
Hauser-Kraus Annulation
General Characteristics The synthesis of naphthalene hydroquinones from phthalide anions and α,β–undaturated carbonyl compounds through the Michael addition-then-Dieckmann condensation is known as ...
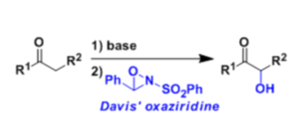
Davis Oxidation
General Characteristics Ketones and esters can be hydroxylated at the α-position when their enolates are treated with 2-sulfonyloxaziridine (N-sulfonyloxaziridine, the Davis reagent). Asymmetric ...
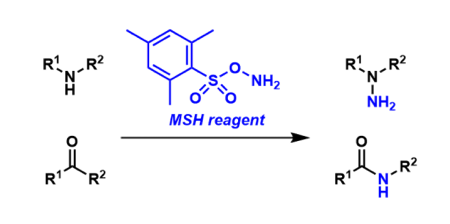
MSH Reagent
General Characteristics MSH (O-(mesitylsulfonyl)hydroxylamine) is an electrophilic aminating agent used to introduce a NH2 unit. It is used in reactions such as the Beckmann rearrangement, the Neber ...
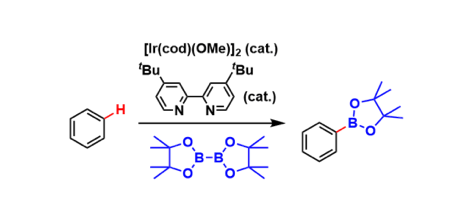
Hartwig-Miyaura C-H Borylation
General Characteristics Iridium catalysts coordinated with electron-donating bidentate ligands promote direct C-H borylation of non-halogenated aromatic rings. Organoboronate compounds are, of ...
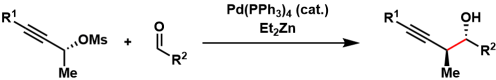
Marshall Propargylation
General Characteristics In the Marshall propargylation, propargyl mesylates and aldehydes are reductively coupled to form a new carbon-carbon bond. The transfer of chirality from the mesylates ...