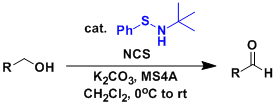
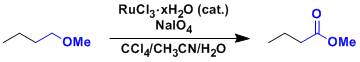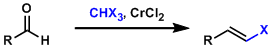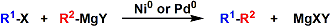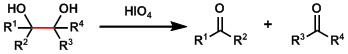General Characteristics The Swern oxidation is relatively clean and practical but also associated with such problems as the generation of carbon monoxide and dimethylsulfide and the requirement of ...
Author Archive
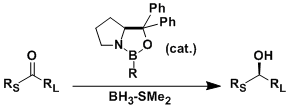
Corey-Bakshi-Shibata (CBS) Reduction
General Characteristics The asymmetric reduction of ketones catalyzed by the chiral oxazaborolidine (derived from proline) is called the Corey-Bakshi-Shibata (CBS) reduction. The reaction has a wide ...
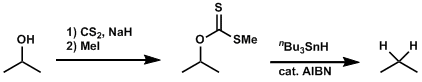
Barton-McCombie Deoxygenation
General Characteristics The free radical-mediated deoxygenation of alcohol compounds after the formation of thiocarbonyl intermediates is known as the Barton-MacCombie deoxygenation. This reaction is ...
Ruthenium Tetroxide (RuO4)
General Characteristics Ruthenium tetroxide is a very strong oxidizing agent capable of even oxidative cleavage of benzene rings and olefins. The reagent can therefore be thought of as an alternative ...
Takai-Uchimoto Olefination
General Characteristics -The synthesis of alkenyl halides from aldehydes using the gem-dichromium reagent prepared from haloform (CHX3) and CrCl2 is known as the Takai-Uchimoto reaction. Mild ...
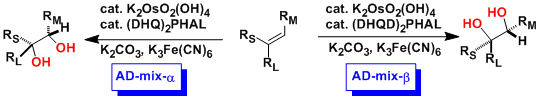
Sharpless Asymmetric Dihydroxylation (Sharpless AD)
General Characteristics -Sharpless developed chiral ligands ((DHQD)2PHAL or (DHQ)2PHAL) derived from cinchona alkaloids (quinidine, quinine) for the practical osmium tetroxide-catalyzed asymmetric ...
Kumada-Tamao-Corriu Cross Coupling
General Characteristics The palladium- or nickel-catalyzed cross coupling between aryl halides/triflates and Grignard reagents is known as the Kumada-Tamao-Corriu reaction. The Kumada-Tamao-Corriu ...
Malaprade Glycol Oxidative Cleavage
General Characteristics Periodic acid or sodium periodate is used to oxidatively cleave 1,2-diols, producing a pair of aldehydes or ketones. Sodium periodate also works as the reoxidant of osmium ...
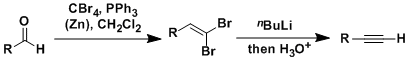
Corey-Fuchs Alkyne Synthesis
General Characteristics The Corey-Fuchs reaction is the conversion of aldehydes into alkynes with one carbon homologation. The treatment of 1,1-dihaloalkenes with 2 equivalents of alkyllithium gives ...
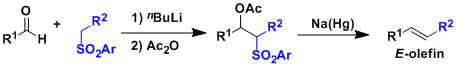
Julia-Lythgoe Olefination
General Characteristics The Julia-Lythgoe olefination involves the nucleophilic addition of lithiosulfones to carbonyl compounds, acylation, and reductive treatment of the resulting ...