Nambo, M.; Crudden, C. M. Angew. Chem. Int. Ed. 2013, Early view.
DOI: 10.1002/anie.201307019
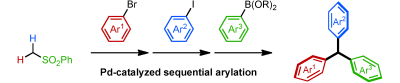
Triarylmethanes, which are valuable structures in materials, sensing and pharmaceuticals, have been synthesized starting from methyl phenyl sulfone as an inexpensive and readily available template. The three aryl groups were installed through two sequential palladium-catalyzed C–H arylation reactions, followed by an arylative desulfonation. This method provides a new synthetic approach to multisubstituted triaryl- methanes using readily available haloarenes and aryl boronic acids, and is also valuable for the preparation of unexplored triarylmethane-based materials and pharmaceuticals.
Unsymmetric triarylmethanes have been synthesized starting from methyl phenyl sulfone as an inexpensive and readily available template. The three aryl groups were installed through two sequential palladium-catalyzed C-H arylation reactions, followed by an arylative desulfonation.
Prof Crudden have double affiliation on Institute of Transformative Bio-Molecles, Nagoya University as well as on Queen’s University at Canada. This work was conducted in Nagoya University with Co-PI, Dr Masakazu Namo.
[amazonjs asin=”3527332790″ locale=”US” title=”Transition Metal-Catalyzed Couplings in Process Chemistry: Case Studies From the Pharmaceutical Industry”][amazonjs asin=”3540329587″ locale=”US” title=”Metal Catalyzed Cascade Reactions (Topics in Organometallic Chemistry)”]