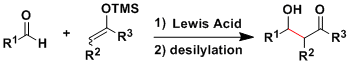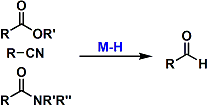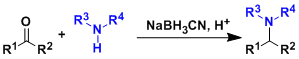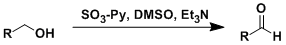For a moment, the world was astonished. But looking beyond the surface, it was something that was not clear. The STAP Cell fever[1] is an issue that has cast the spotlight on the problems that ...
Monthly Archives: May 2014
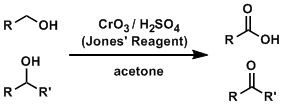
Jones Oxidation
General Characteristics The Jones oxidation is one of the most fundamental reactions used to oxidize alcohol compounds. The reagents do not react with alkenes and alkynes and oxidize only alcohols. ...
Mukaiyama Aldol Reaction
General Characteristics Acid- or base-promoted classical aldol reactions give a complex mixture of products because of the reversibility and the difficulty of controlling the enolate geometry. In the ...
Partial Reduction of Esters, Amides, and Nitriles with Metal Hydride Reagents
General Characteristics Esters, amides, and nitriles are reduced to aldehydes (without overreduction) using appropriate metal hydride reagents at low temperatures. DIBAL and Red-Al are used commonly ...
Borch Reductive Amination
General Characteristics The reaction of aldehydes or ketones with ammonia equivalents or primary or secondary amines in the presence of hydride reducing agents gives alkylated amines. The reductive ...
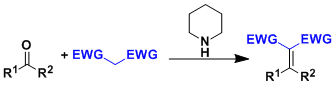
Knoevenagel Condensation
General Characteristics Activated methylene compounds condense with aldehydes and ketones to give substituted alkenes. Piperidine is generally used as the catalyst. Nitromethane also undergoes ...
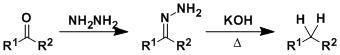
Wolff-Kishner Reduction
General Characteristics The deoxygenation of carbonyl compounds through hydrazone formation is known as the Wolff-Kishner reduction. Heating a mixture containing an aldehyde or a ketone, hydrazine, ...
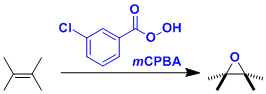
Prilezhaev Epoxidation
General Characteristics The oxidation of olefins to epoxides using peracids is known as the Prilezhaev epoxidation. m-Chloroperoxybenzoic acid (mCPBA) in particular is used often in laboratories ...
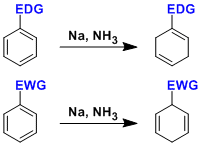
Birch Reduction
General Characteristics -Aromatic compounds are reduced to 1,4-cyclohexadienes by treatment with alkali matals (Li, Na, or K) or alkaline earth metals (Ca or Mg) dissolved in a mixed solvent of ...
Parikh-Doering Oxidation
General Characteristics The Parikh-Doering oxidation uses SO3•Py (a solid reagent that can be handled easily) as the activator of DMSO. This reaction is especially effective for the oxidation of ...