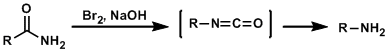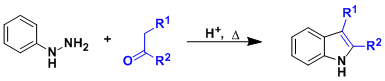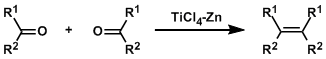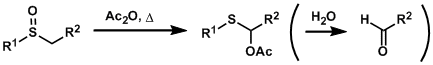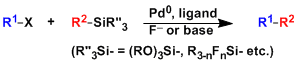General Characteristics In the Hofmann rearrangement, primary amides rearrange into isocyanates upon treatment with halogens and bases and are ultimately converted into primary amines with one-carbon ...
Posts by Category: Reactions
Wolff Rearrangement
General Characteristics The rearrangement of the carbenes formed by elimination of nitrogen from diazoketones giving ketenes is known as the Wolff rearrangement. The ketenes are trapped by ...
Mukaiyama Redox Condensation
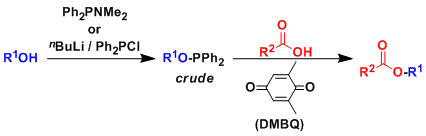
General Characteristics The quinone-phosphine redox system can be used to activate alcohols towards esterification, etherification, and other SN2 reactions with various nucleophiles. The reaction is ...
Fischer Indole Synthesis
General Characteristics Upon heating under acidic conditions, aryl hydrazones synthesized from aldehydes/ketones and aryl hydrazines undergo sigmatropic rearrangement and aromatization to give ...
Pfitzner-Moffatt Oxidation
General Characteristics The oxidation of alcohols using the combination of DCC+Brønsted acid+DMSO is known as the Pfitzner-Moffatt oxidation. The active species of this reaction is bulkier than those ...
McMurry Coupling
General Characteristics In the McMurry reaction, alkenes are synthesized by the reductive coupling of aldehydes and ketones using low valent titanium reagents. This reaction can be thought of as the ...
Pummerer Rearrangement
General Characteristics The treatment of sulfoxides with acid anhydrides or acid chlorides brings about a reaction called the Pummerer rearrangement, which gives α-acyloxythioethers. The rearranged ...
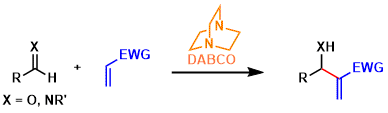
Morita-Baylis-Hillman Reaction
General Characteristics The Morita-Baylis-Hillman reaction is a type of carbon-carbon bond forming reactions between aldehydes/imines and electron deficient alkenes promoted by nucleophilic ...
Hiyama Cross Coupling
General Characteristics The cross coupling reaction between aryl halides/triflates and organosilicon reagents (organosilanes) is generally called the Hiyama coupling. Most organosilicon reagents are ...
Ether Synthesis by Acetal Reduction
General Characteristics The reduction of the oxonium cations generated by acidic treatment of acetals is a way to synthesize ethers. Triethylsilane (Et3SiH) is a reducing agent that is stable under ...