Uesugi, S.; Li, Z.; Yazaki, R.; Ohshima, T. Angew. Chem., Int. Ed. 2014. Early View.
DOI: 10.1002/anie.201309755
A highly chemoselective conjugate addition of alcohols in the presence of amines is described. The cooperative nature of the catalyst enabled chemoselective activation of alcohols over amines, allowing the conjugate addition to soft Lewis basic α,β-unsaturated nitriles. Divergent transformation of the nitrile functionality highlights the utility of the present catalysis.
Achieving high levels of chemoselectivity has been the Achilles’ heel of chemical synthesis.[1] Previously, Ohshima and Mashima reported chemoselective reactions of hydroxy groups in the presence of an amino group by using Zn-cluster (ZnTAC24) or Co-cluster Co2(OCOtBu)2-(bpy)2(μ2-OCH2-C6H4-4-CH3)2 as catalysts.[2]
In this paper, Ohshima and coworkers reported the first example of a catalyst-controlled chemoselective conjugate addition of a hydroxy group in the presence of an amino group. Using commercially available copper(I) 3-methylsalicylate/dppe was effective catalyst, the Conjugate addition to acrylonitrile and it derivatives in the presence of mixtures of structurally similar amine and alcohols to deliver O-adduct in high yield with high chemoselectivity.
-
References
[1] Afagh, N. A.; Yudin, A. K. Angew. Chem., Int. Ed. 2010, 49, 262. DOI: 10.1002/anie.200901317
[2] (a) “Enzyme-Like Chemoselective Acylation of Alcohols in the Presence of Amines Catalyzed by a Tetranuclear Zinc Cluster”
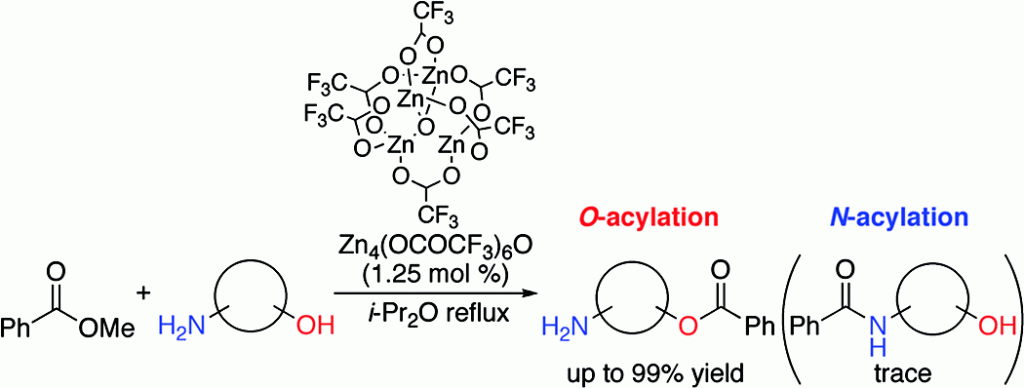
Ohshima, T.; Iwasaki, T.; Maegawa, Y.; Yoshiyama, A.; Mashima, K. J Am Chem Soc 2008, 130, 2944. DOI: 10.1021/ja711349r
Acylation of alcohols and amines is one of the most fundamental reactions. Due to the greater nucleophilicity of the amino group compared to the hydroxyl group, complete N-acylation occurs. Only an enzymatic reaction can promote a highly selective O-acylation reaction, and there are no examples using an artificial catalyst. Here we report that the tetranuclear zinc cluster Zn4(OCOCF3)6O efficiently catalyzes highly chemoselective O-acylation in the presence of primary and secondary alkyl amines. Our results suggest the high potential of the zinc cluster as the core structure of an artificial enzyme to realize further enzyme-like chemoselective reactions.
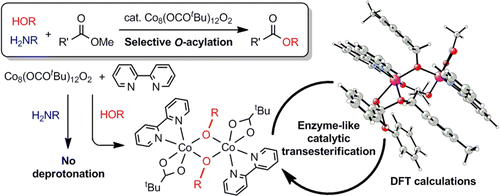
[2] “Enzyme-Like Catalysis via Ternary Complex Mechanism: Alkoxy- Bridged Dinuclear Cobalt Complex Mediates Chemoselective O‐Esterification over N‐Amidation”
Hydroxy group-selective acylation in the presence of more nucleophilic amines was achieved using acetates of first-row late transition metals, such as Mn, Fe, Co, Cu, and Zn. Among them, cobalt(II) acetate was the best catalyst in terms of reactivity and selectivity. The combination of an octanuclear cobalt carboxylate cluster [Co4(OCOR)6O]2 (2a: R = CF3, 2b: R = CH3, 2c: R = tBu) with nitrogen-containing ligands, such as 2,2′-bipyridine, provided an efficient catalytic system for transesterification, in which an alkoxide-bridged dinuclear complex, Co2(OCOtBu)2(bpy)2(μ2-OCH2-C6H4-4-CH3)2 (10), was successfully isolated as a key intermediate. Kinetic studies and density functional theory calculations revealed Michaelis–Menten behavior of the complex 10 through an ordered ternary complex mechanism similar to dinuclear metallo-enzymes, suggesting the formation of alkoxides followed by coordination of the ester.
-
Related Links
Ohshima group, Kyushu University
-
Related Books
[amazonjs asin=”3527333754″ locale=”US” title=”Directed Selectivity in Organic Synthesis: A Practical Guide”][amazonjs asin=”0080370675″ locale=”US” title=”Conjugate Addition Reactions in Organic Synthesis (Tetrahedron Organic Chemistry)”]