Iwasaki, K.; Wan, K. K.; Oppedisano, A.; Crossley, S. W. M.; Shenvi, R. A. J. Am. Chem. Soc. 2014, ASAP.
DOI: 10.1021/ja412342g
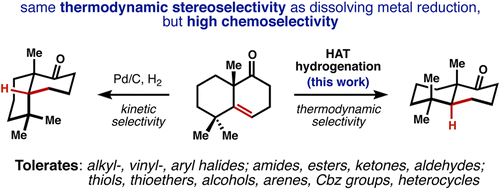
Few methods permit the hydrogenation of alkenes to a thermodynamically favored configuration when steric effects dictate the alternative trajectory of hydrogen delivery. Dissolving metal reduction achieves this control, but with extremely low functional group tolerance. Here we demonstrate a catalytic hydrogenation of alkenes that affords the thermodynamic alkane products with remarkably broad functional group compatibility and rapid reaction rates at standard temperature and pressure.
Hydrogenation of Alkenes – addition of H-H (H2) to the π-bond of alkenes to afford an alkane. The reaction must be catalyzed by metals such as Pd, Pt, Rh, and Ni.
The catalytic hydrogenation that take place at the “less hindered” side of a reactant are common in organic chemistry and are examples of steric effects on reactivity. Dissolving metal reduction provides a means to achieve thermodynamic control and can reduce only conjugated or electron-poor alkenes at low temperature, but with extremely low functional group tolerance. Shenvi and coworkers has developed new method for the thermodynamic hydrogenation of alkenes (Mn(dmp)3, PhSiH3, TBHP, i-PrOH, RT).
- Related Link
Shenvi group