X, Cui.; J, Zhao.; Y, Zhou.; J, Ma.; Y, Zhao.; J. Am. Chem. Soc., 2014, 136, 9256–9259
DOI: 10.1021/ja504211y
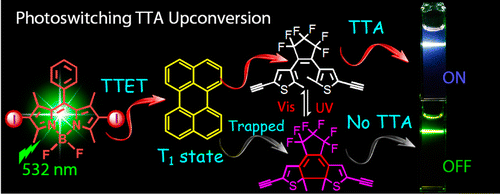
Reversible photoswitched triplet−triplet annihilation upconversion (TTA UC) was demonstrated with dithienylethene (DTE) derivatives as the photo- chromic units, 2,6-diiodoBodipy as the triplet photo- sensitizer, and perylene as the triplet acceptor/emitter. The TTA UC is undisturbed by the open-form DTE but can be switched OFF upon photoirradiation of the mixture of the three components at 254 nm, i.e., by the closed- form DTE. Subsequent visible light irradiation restores the TTA UC. By studying the competitive triplet-state energy- transfer processes with nanosecond time-resolved transient difference absorption and fluorescence spectroscopy, we confirmed that the quenching of the perylene triplet excited state by closed-form DTE is dominant among the four possible quenching processes.
Zhao group from Dalian University of Technology, China reported ” to J. Am. Chem. Soc. about reversible photoswitching. of triplet−triplet annihilation.
Triplet−triplet annihilation up-conversion (TTA UC)[1] is one of the most attractive photo-physical properties because fluorescent molecules endowed with this unique characteristics is capable of converting input low energy light (long wavelength) to high energy light source (shorter wavelength). The author of this paper for the first time achieved controlling the fluorescence ON/OFF switch of (TTA UC) using photochromic molecule. In addition, detail kinetics mechanics of (TTA UC) were also explored and characterized.
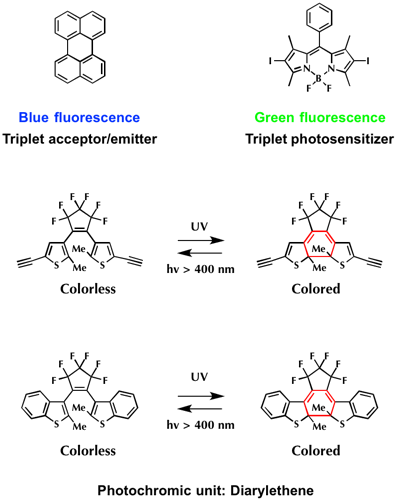
Firstly, perylene (T-state energy = ca. 1.53 eV) and 2,6-diiodoBodipy (T-state energy = ca. 1.69 eV) were each chosen as triplet acceptor/emitter and triplet photosensitized.[2] Next, diarylethenes were selected as photochromic unt. The reason is because they not only show efficient photo-sensitivity but also possess corresponding triplet-state energy levels ca. 1.97 in open-ring form and 1.23 eV in closed-ring form.
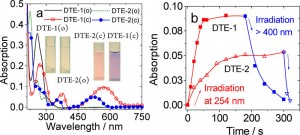
To begin with, the absorption spectrum and switching efficiency of two diarylethens were examined. As shown below, absorption maximum of diarylethene bearing two alkynes DTE-1(c) is red shifted compared to that of benzothiophene based diarylethene DTE-2(c). Accordingly, the photo-switching efficiency of DTE-1(c) is less sensitive than that of DTE-2(c).
<Up-conversion emission and its photo-switching>
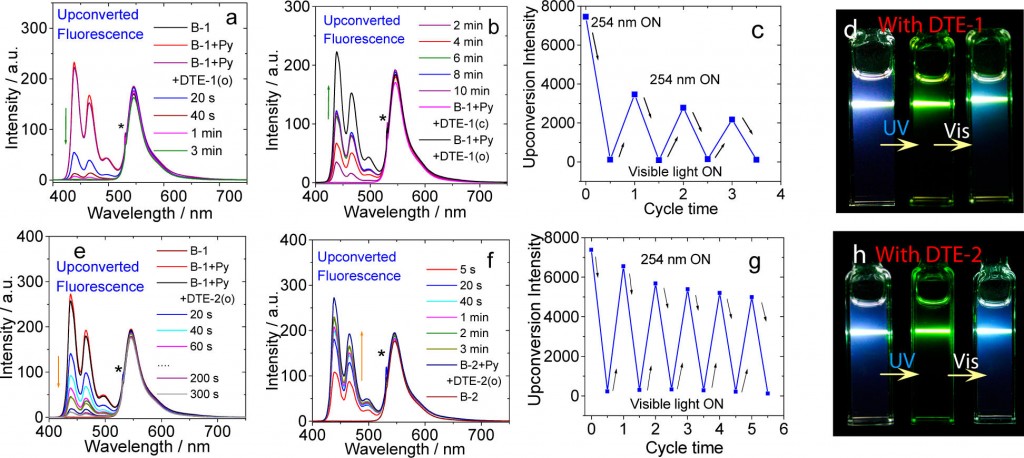
Blue up conversion fluorescence(around 440 nm emission maximum) from perylene was observed upon mixing perylene and 2,6-diiodoBodipy using 532 nm laser. Additional adding Diarylethene (DTE-1(o) and DTE-2(o)) followed by irradiation of 254 nm laser successfully bleached the up conversion fluorescence from perylene (Figure a and e). Conversely, up conversion fluorescence from perylene get recovered by irradiating visible light to the cell (Figure a and e). This is responsible for the photochromic reaction of the diarylethene(DTE-1(o) and DTE-2(o)) switched back to the initial state: (DTE-1(c) and DTE-2(c)) respectively. This photo-switching can be repeated many times as shown below (Figure c and d).
<Detail mechanism>
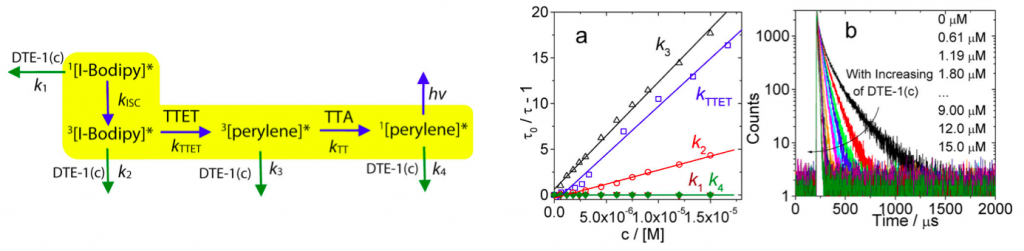
To shed light on the mechanism of photoswitching TTA UC, the excited state fluorescence quenching constants at each possible transition states (left figure) were determined. Steady-state and nanosecond/picosecond time-resolved spectroscopy were used to determine these constants. Finally, as shown in (figure a and c), it was revealed that the diarylethene(closed-form) served as fluorescence accepter of delayed fluorescence from the perylene therefore was the main cause of these dramatic fluorescence.[3]
- Reference
[1] “Photon upconversion based on sensitized triplet–triplet annihilation”
Singh-Rachford, T. N.; Castellano, F. N. Coord. Chem. Rev. 2010, 254, 2560−2573. DOI: 10.1016/j.ccr.2010.01.003
Photon upconversion, the process wherein light of long wavelength is frequency converted to photons of higher energy, is readily achieved at low incident power through sensitized triplet–triplet annihilation (TTA) in various chromophore combinations spanning the UV to the near-IR. This emerging wavelength-shifting technology truly represents a viable route towards converting low energy terrestrial solar photons into light adequate to drive electron transfer in operational photovoltaics. Generalized molecular design constraints, all operational examples reported to date, and measurement techniques applied to these low power nonlinear processes are reviewed in this contribution. In many instances, direct visualization of this phenomenon is presented in solution and within various polymeric host materials.
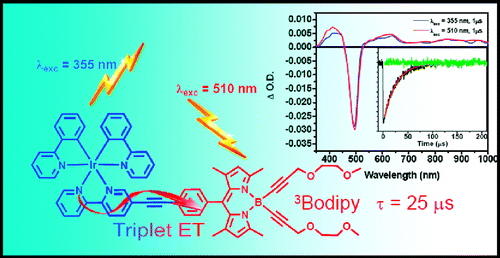
[2] “Boron Dipyrromethene (Bodipy) Phosphorescence Revealed in [Ir(ppy)2(bpy-C≡C-Bodipy)]+”
Rachford, A. A.; Ziessel, R.; Bura, T.; Retailleau, P.; Castellano, F. N. Inorg. Chem. 2010, 49, 3730−3736. DOI: 10.1021/ic901996u
The synthesis, structural characterization, electrochemistry, and molecular photophysics of [Ir(ppy)2(bpy-C≡C-Bodipy)](PF6), where ppy is 2-phenylpyridine and bpy-C≡C-Bodipy is 5-ethynyl-2,2′-bipyridine-8-phenyl-1,3,5,7-tetramethyl-4,4-bis(2,5-dioxaoct-7-ynyl)-4-bora-3a,4a-diaza-s-indacene (4), is presented. Static and dynamic photoluminescence and absorption measurements in conjunction with cyclic voltammetry were employed to elucidate the nature of the intramolecular energy transfer processes occurring in the excited state of the title chromophore. Parallel studies were performed on appropriate model chromophores (2 and 3) intended to represent the photophysics of the isolated molecular subunits, that is, triplet metal-to-ligand-charge-transfer (3MLCT) and triplet Bodipy intraligand (3IL) excited states, respectively. Upon charge transfer excitation of the title chromophore, the 3MLCT based phosphorescence readily observed in 2 (Φem = 0.027, τ = 243 ns) is quantitatively quenched resulting from production of the 3Bodipy excited state through intramolecular triplet−triplet energy transfer. The formation of the 3Bodipy-localized excited state is confirmed by features in the transient absorption difference spectrum, extended excited-state lifetime (τ = 25 μs), as well the observation of 3IL Bodipy-based phosphorescence detected at 730 nm at 77 K. The low temperature Bodipy phosphorescence is readily produced in 4 as a result of the internal Ir(III) heavy atom.
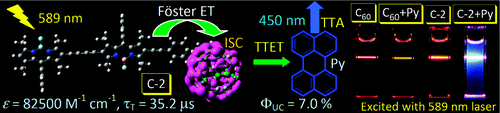
[3] “Light-Harvesting Fullerene Dyads as Organic Triplet Photosensitizers for Triplet–Triplet Annihilation Upconversions”
Wu, W.; Zhao, J.; Sun, J.; Guo, S. J. Org. Chem. 2012, 77, 5305− 5302. DOI: 10.1021/jo300613g
Visible light-harvesting C60–bodipy dyads were devised as universal organic triplet photosensitizers for triplet–triplet annihilation (TTA) upconversion. The antennas in the dyad were used to harvest the excitation energy, and then the singlet excited state of C60 will be populated via the intramolecular energy transfer from the antenna to C60 unit. In turn with the intrinsic intersystem crossing (ISC) of the C60, the triplet excited state of the C60 will be produced. Thus, without any heavy atoms, the triplet excited states of organic dyads are populated upon photoexcitation. Different from C60, the dyads show strong absorption of visible light at 515 nm (C-1, ε = 70400 M–1 cm–1) or 590 nm (C-2, ε = 82500 M–1 cm–1). Efficient intramolecular energy transfer from the bodipy moieties to C60 unit and localization of the triplet excited state on C60 were confirmed by steady-state and time-resolved spectroscopy as well as DFT calculations. The dyads were used as triplet photosensitizers for TTA upconversion, and an upconversion quantum yield up to 7.0% was observed. We propose that C60–organic chromophore dyads can be used as a general molecular structural motif for organic triplet photosensitizers, which can be used for photocatalysis, photodynamic therapy, and TTA upconversions.
- Related Books
[amazonjs asin=”0306458829″ locale=”US” title=”Organic Photochromic and Thermochromic Compounds: Main Photochromic Families (Topics in Applied Chemistry)”]