Ishida, S.; Hirakawa, F.; Frukawa, K.; Yoza, K.; Iwamoto, T. Angew. Chem. Int. Ed. 53, 2014. Early View.
DOI: 10.1002/anie.201405509
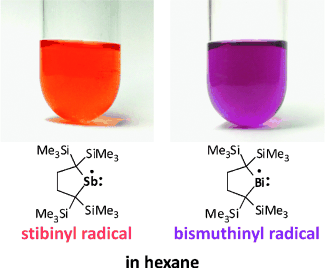
Stibinyl and bismuthinyl radicals are recognized as representative intermediates of antimony and bismuth compounds, but still elusive in the condensed phase. We successfully synthesized persistent stibinyl and bismuthinyl radicals in solution by facile dissociation of the corresponding dimers with bulky substituents. We characterized the radicals by NMR and UV/Vis spectroscopy and estimated the thermodynamic parameters for the dissociation equilibria. The radicals show n→p (HOMO→SOMO) transition bands at 497 nm (stibinyl) and 543 nm (bismuthinyl) in 3-methylpentane and react with a stable nitroxyl radical.
Neutral dicoordinate antimony- and bismuth radicals can be generated in situ by the homolytic cleavage of the weak Pn-R bond in R3Pn derivatives (Pn=Sb, Bi). They are sometimes recognized as key intermediates in organic synthesis, polymerization processes, and organometallic chemical vapor deposition processes.
In marked contrast to the comprehensive study of various stable phosphinyl and arsinyl radicals, however, the corresponding isolable heavier analogues involving Sb and Bi radicals are still extremely rare.
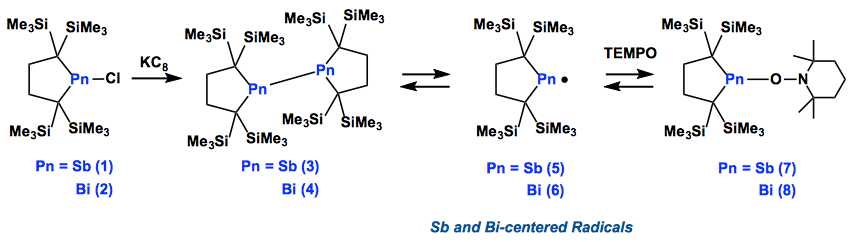
In 2011, Iwamoto group has successfully isolate a novel dialkylphosphinyl radical by employing the bulky [H2C(Me3Si)2C–]2 ligand, which also allowed them to synthesize a dialkylsilylene before.[1,2] Using the same ligand, Iwamoto at al. prepared distibine 3and dibismuthine 4.
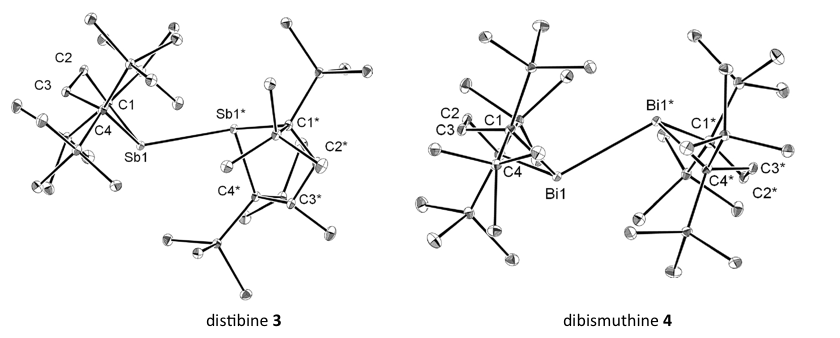
Of course, they are closed shell species and NMR active (EPR silent). However, Iwamoto et al. found that these compounds (3,4 ) possess extraordinarily long Pn-Pn bonds in their solid state and readily dissociate reversibly into the corresponding radicals (5,6) in solution, which was confirmed by variation temperature NMR and UV/Vis spectroscopy analyses as well as theoretical studies. These radicals were trapped chemically with TEMPO, which afforded cross-radical coupling products (7, 8).
References
[1] “A Stable Dialkylphosphinyl Radical”
Ishida, S.; Hirakawa, F.; Iwamoto, T. J. Am. Chem. Soc. 2011, 133, 12968-12971. DOI: 10.1021/ja205001m
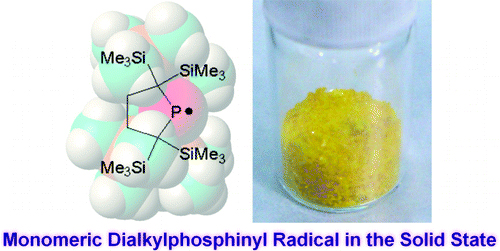
Dialkylphosphinyl radical 1was synthesized as thermally stable yellow crystals and found to be monomeric both in solution and in the solid state. EPR spectrum showed that the spin density of 1 is mainly localized on the 3p orbital of the dicoordinated phosphorus atom. A distinct absorption band due to the electronic transition from nonbonding electron pair orbital to singly occupied 3p orbital on the phosphorus atom of 1was observed at 445 nm in solution. Phosphinyl radical 1 underwent facile reaction with carbon tetrachloride, hydrogen abstraction, and a unique reaction with a persistent radical, galvinoxyl, giving a cyclic phosphaalkene and a silylether.
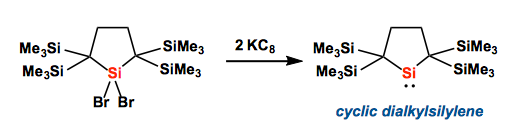
[2] “The First Isolable Dialkylsilylene”
Kira, M.; Ishida, S.; Iwamoto, T.; Kabuto, C. J. Am. Chem. Soc. 1999, 121, 9722 – 9723. DOI: 10.1021/ja9925305
The first isolable dialkylsilylene, 2,2,5,5-tetrakis(trimethylsilyl)silacyclopentane-1,1-diyl 1, which is well protected sterically from dimerization but least perturbed electronically; the intrinsic properties of silylene are entirely embodied in 1. Interestingly, 1 is storable at 0 °C in the solid state but isomerizes slowly to the corresponding silaethene via an apparent 1,2-silyl migration at room temperature in solution.
Related Links