Yang, Y.; Hughes, R. P.; Aprahamian, I. J. Am. Chem. Soc. 2014, 136 ,13190–13193
DOI: 10.1021/ja508125n
ABSTRACT: Increasing the electron density in BF2- coodinated azo compounds through para-substitution leads to a bathochromic shift in their activation wave- length. When the substituent is dimethyl amine, or the like, the trans/cis isomerization process can be efficiently modulated using near infrared light. The electron donating capability of the substituent also controls the hydrolysis half-life of the switch in aqueous solution, which is drastically longer for the cis isomer, while the BF2- coodination prevents reduction by glutathione.
Introduction
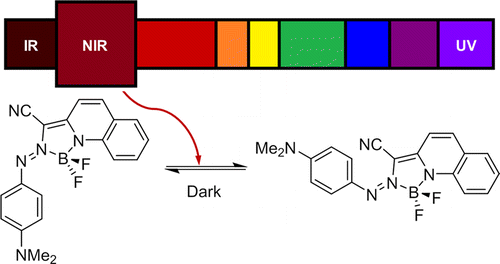
Photochromic azo derivatives are oldest light driven molecular switch in the history of photochemistry. Unaccountable research has been done in search of more sophisticated azo-derivatives. Considering practical application in many fields, one promising property to be achieved is to shift the absorption into near-infrared (NIR) region. Based on their previous research[1] and computational calculation, Aprahamian in Dartmouth college, further modified BF2-fused azo system to achieve various NIR photoswitchable derivatives.[2]
Molecular structure and spectrum
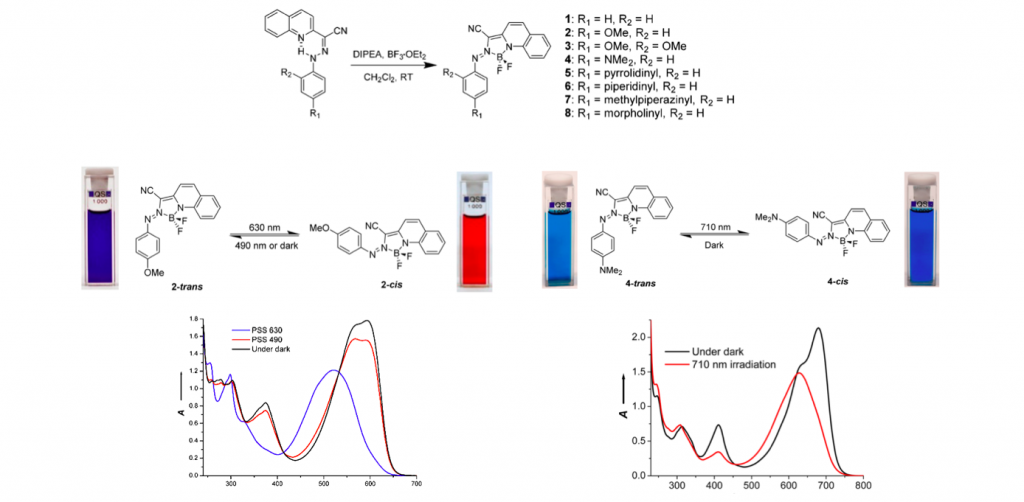
Based on their own synthetic strategy, various para-functionalized derivatives were prepared in hope of further shift the absorption region to NIR. Although not detailed here, the synthesized derivatives exhibited good photoresponsivity and more importantly absorb NIR light as shown below.
Reference
(1) Smith, K. C., Ed. The Science of Photobiology; Plenum Press: New York, 1977.
(2)” Visible Light Switching of a BF2‐Coordinated Azo Compound” Yang, Y.; Hughes, R. P.; Aprahamian, I. J. Am. Chem. Soc. 2012, 134, 15221−15224.
DOI: 10.1021/ja306030d