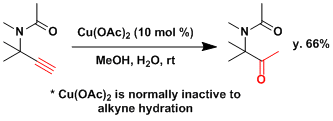- Popularity
- Reliability
- Criteria #3
- Criteria #4
- Criteria #5
-
Characteristics
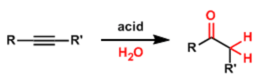
Hydration of alkynes is simply the addition of H2O in the presence of Lewis acids. The hydration of alkynes has been extensively studied for more than 100 years. This reation allows access to various carbonyl derivatives starting from alkynes. The hydration of activated alkynes follows Markovnikov’s rule, so only acetylene gives an aldehyde.
For a long time the hydration of acetylene has been a reaction of a major industrial interest. In fact, before the advent of the Wacker process, acetaldehyde was essentially produced by the hydration of acetylene. Since the discovery of acceleration of alkyne hydration in the presence of mercury(II) ions (Kucherov reaction), a wide variety of mercury(II) compounds have been employed as catalysts, mainly in aqueous acid solutions. It is nowadays possible to i) control the regiochemistry of various additions of nucleophiles ii) more mild conditions to alkynes by applying different transition-metal catalysts (See in example of reactions).
-
Literature reference
・Kutscheroff, M. Chem. Ber. 1881, 14, 1540.
・Kutscheroff, M. Chem. Ber. 1884, 17, 13.
・Thomas, R. J.; Campbell, K. N.; Hennion, G. F. J. Am. Chem. Soc. 1938, 60, 718. DOI:10.1021/ja01270a061
<Review>
・Beller, M.; Seayad, J.; Tillack, A.; Jiao, H. Angew. Chem. Int. Ed. 2004, 43, 3368. DOI:10.1002/anie.200300616
・Alonso, F.; Beletskaya, I. P.; Yus, M. Chem. Rev. 2004, 104, 3079. DOI: 10.1021/cr0201068
・Hinterman, .; Labonne, A. Synthesis 2007, 1121. DOI: 10.1055/s-2007-966002
-
Reaction mechanism
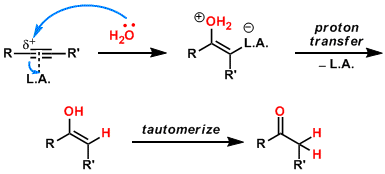
The activation of the carbon-carbon triple bond toward nucleophilic attack of water can be performed by metals with high Lewis acidity (alkyne activation). It is easy to realize that as a result of the anti-addition of the nucleophile, the product should be obtained with trans-stereochemistry. In the case of water as the nucleophile, Proton transfer from O to C converts enol to more stable keto tautomer.
However, in many other cases the picture is quite different, the reactions proceeding as a result of the insertion of the alkyne moiety into M-H or M-Nu bonds of “H- M-Nu” complexes, typically with syn-addition (nu- cleophile activation).
-
Example of reactions
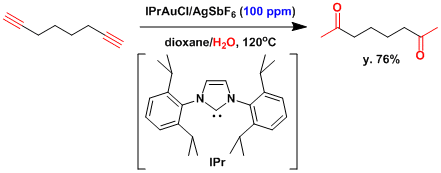
Nolan and coworkers reported the acid-free catalyzed alkyne hydration using a gold catalyst.[1] Using a gold-NHC complex and silver hexafluoroantimonate, the authors demonstrated the outstanding activity of this catalyst in the hydration of various alkynes using 1,4-dioxane and methanol as reaction media.
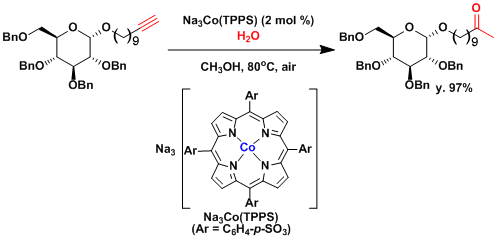
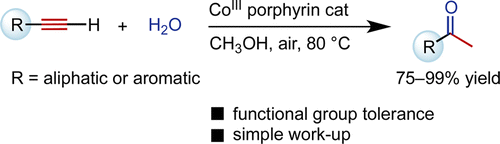
Naka and coworkers have been demonstrated the hydration of terminal alkynes by using water-soluble cobalt(III) porphyrin complexes[2] The catalysis can be performed under near-neutral conditions that are compatible with the presence of acid-labile functional groups.
As it has already been described above, the addition of water across a triple bond generally follows the Markovnikov rule. On the other hand, an anti-Markovnikov hydration of terminal alkynes could be a convenient way to the preparation of aldehydes, but ruthenium complexes have been identified which allowed the catalysis of this unusual hydration mode.[3] The first reports by Tokunaga and Wakatsuki using ruthenium complexes, who disclosed Ru(IV)-vinylidene species as the key intermediates in 1998.[3a]
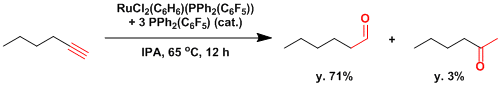
Effective regioselective hydration is facilitated by the neighboring carbonyl group.[4]
-
Bibliography
[1] Marion, N.; Ramon, R. S.; Nolan, S. P. J. Am. Chem. Soc. 2009, 131, 448. DOI: 10.1021/ja809403e
[2] “Hydration of Terminal Alkynes Catalyzed by Water-Soluble Cobalt Porphyrin Complexes”
Tachinami, T.; Nishimura, T.; Ushimaru, R.; Noyori, R.; Naka, H. J. Am. Chem. Soc. 2013, 135, 50, DOI: 10.1021/ja310282t
Water-soluble cobalt(III) porphyrin complexes were found to promote the hydration of terminal alkynes to give methyl ketones. The alkyne hydration proceeded in good to excellent yield with 0.1 to 2 mol % cobalt catalyst 1 and was compatible with the presence of acid/base- or redox-sensitive functional groups such as alkyl silyl ethers; allyl ethers; trityl ethers; benzyl ethers; carboxylic esters; boronic esters; carboxamides; nitriles; and nitro, iodo, and acetal groups. Some of the alkyne substrates tested here are otherwise difficult to hydrate. The alkyne hydration can be performed on a gram scale, and the catalyst can be recovered by aqueous workup.
[3] (a) Tokunaga, M.; Wakatsuki, Y. Angew. Chem. Int. Ed. 1998, 37, 2867.[abstract] (b) Suzuki, T.; Tokunaga, M.; Wakatsuki, Y. Org Lett 2001. 3. 735. DOI: 10.1021/ol0003937
[4] Easton, N. R.; Cassady, D. R.; Dillard, R. D. J. Org. Chem. 1965, 30, 3084. DOI:10.1021/jo01020a048
-
Related Links
・Synthesis of ketones by hydration of alkyne (organic-chemistry.org) ・Addition Reaction of Alkynes