S, Yagai.; S, Okamura.; Y, Nakano.; M, Yamauchi.; K, Kishikawa.; T, Karatsu.; A, Kitamura.; A, Ueno.; D, Kuzuhara.; H, Yamada.; T, Seki.; H, Ito. Nature comm., 2014, 5, 4013.
DOI: 10.1038/ncomms5013
p-Conjugated compounds that exhibit tunable luminescence in the solid state under external mechanical stimuli have potential applications in sensors and imaging devices. However, no rational designs have been proposed that impart these mechano-responsive luminescent properties to p-conjugated compounds. Here we demonstrate a strategy for mechano- responsive luminescent materials by imparting amphiphilic and dipolar characteristics to a luminescent p-conjugated system. The oligo(p-phenylenevinylene) luminophore with a didodecylamino group at one end and a tri(ethylene glycol) ester group at the other end yields segregated solid structures by separately aggregating its hydrophobic and hydrophilic moieties. The segregated structures force the molecules to align in the same direction, thereby generating a conflict between the side-chain aggregation and dipolar stabilization of the p-system. Consequently, these metastable solid structures can be transformed through mechanical stimulation to a more stable structure, from a p–p stacked aggregate to a liquid crystal and further to a crystalline phase with variable luminescence.
Yagai group, Chiba University reported a strategy for mechano-responsive luminescent materials by imparting amphiphilic and dipolar characteristics to a luminescent π-conjugated system. Nature Comm. External stimuli responsive material have fascinated scientist for a long time. During past decades, mechanical force like pressing, twisting and bending have been recognized as promising source of another external stimuli.[1] Although various mechanical responsible materials have been reported, the absence of rational blueprint for designing such stimuli-responsive material heavily limits their deeper understanding and further implementation. In this paper, the author demonstrated for the first time the theoretical strategy of designing mechanical responsive materials.
<Molecular design>
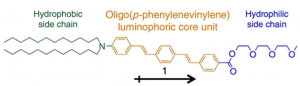
(1) Oligo(p-phenylenevinylene) was selected as core moiety because of its relatively flexible structure and narrow HOMO-LUMO gap.
(2) Electron withdrawing ester and electron donating tertiary amine group were directly attached the terminal position of Oligo(p-phenylenevinylene). This realized the solvatochromism in the visible region thanks to the further narrowed HOMO-LUMO gap and dramatically enhanced dipole moment.
(3) To both electron donating and withdrawing groups, long hydrophobic chain and hydrophilic chain were attached respectively. These long chains can fasten the orientation of the molecule and also make the system to be more vulnerable to the external stimuli applied.
The actual picture of fluorescence in various solvent and detail photo-physical data are all described below.
<Changes upon mechanical stimuli>
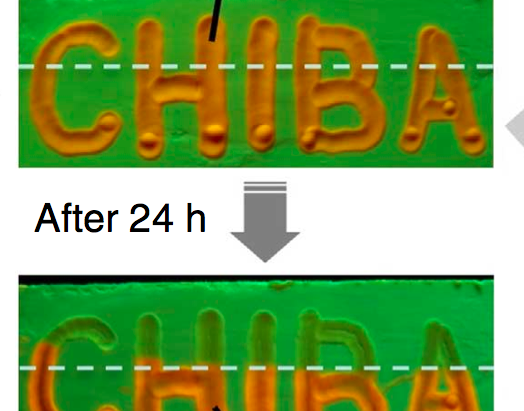
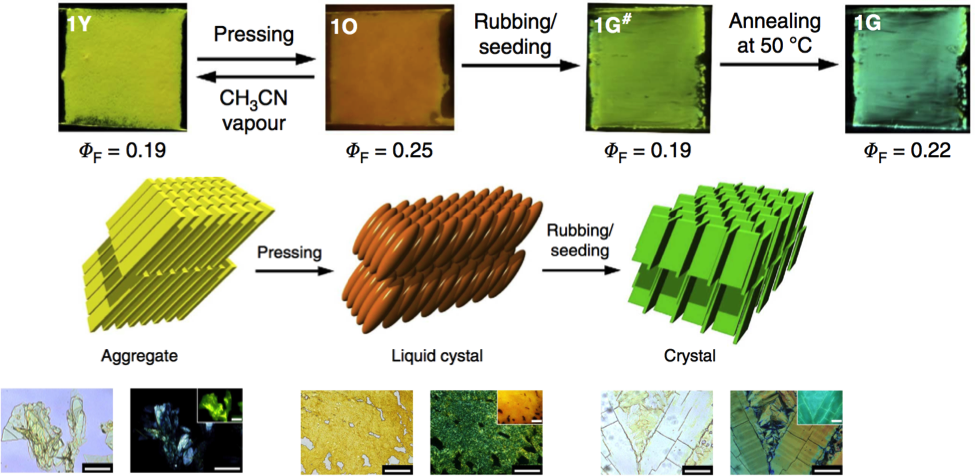
Then the thin film was fabricated from the synthesized solvatochromic molecule. This film, as shown below has four transferable states with different colors. Various experiment like, polarized optical micrograph, fluorescence microscopy observation, powder X-ray diffraction, and scanning electron microscopy were carried out to each four state. These experiments revealed that the initial sheet contains dyes which are tightly π-πstacked and face-to-faced (1Y:Φ= 0.19). After being gentle pressed spontaneous change occurred to whole area, resulting liquid crystal state(1O:Φ= 0.19). Further harsh stimuli, rubbing, to the 1O generate green crystal-form sheet (1G#:Φ= 0.19). Finally, the last state (1G:Φ= 0.22) having shared structure with 1G# is obtained from annealing at 50℃ from 1G.
<Absorption and fluorescence spectrum>
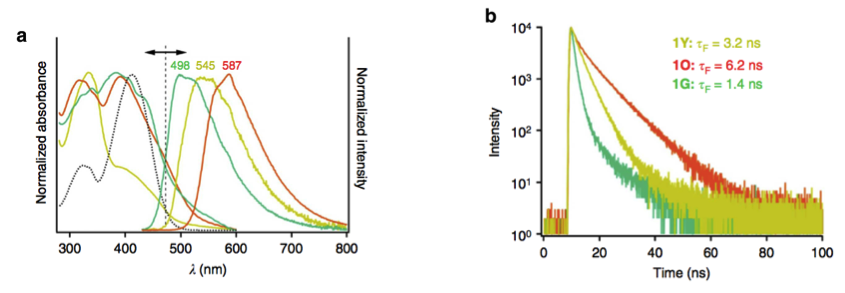
The absorption spectrum showed that the shorter absorption is caused by H-type aggregation. 1O and 1G have almost identical maximum absorptions but different emission spectrum. This similar absorption means 1O and 1G have no interaction in their ground states. In contrast, the different environments in each liquid crystal and crystal state is responsible for the differences in emission spectrum.
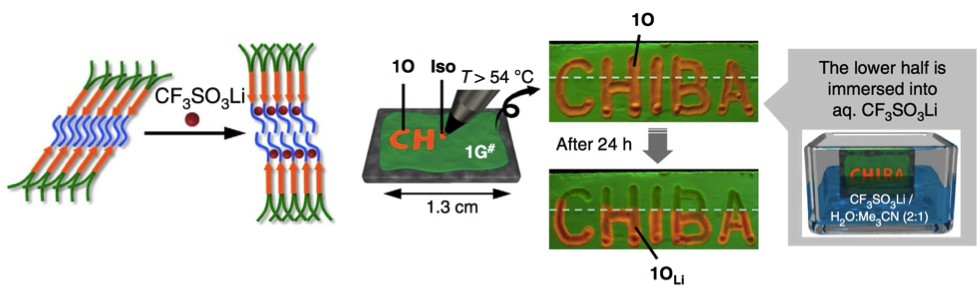
<Controlling the phase changes with lithium ion>
In addition to the mechanical stimuli, the phase change is extremely subjective to the presence of lithium ion presence.[2] The experiment below well demonstrated the usage and further application. As shown below, lithium atoms are entangled with the TEG chain and inhibit the spontaneous formation of 1O from 1G#.
-
References
[1] “Mechanically induced luminescence changes in molecular assemblies”
Sagara, Y.; Kato, T. Nat. Chem. 2009, 1, 605–610. DOI:10.1038/nchem.411
Altering the properties of materials by using an external signal, such as light, heat or mechanical stress, is attractive for the preparation of functional materials in diverse fields. This Perspective focuses on liquid and solid materials that change the colour of their luminescence under mechanical pressure, and highlights the structural changes involved.
[2] “Liquid crystalline assembly of a diblock rod-coil polymer based on poly(ethylene oxide) and its complexes with LiCF3SO3”
Lee, M.; Oh, N.-K.; Lee, H.-K.; Zin, W.-C. Macromolecules 1996, 29, 5567–5573. DOI: 10.1021/ma951418y
The rod−coil polymer of ethyl 4-[4‘-oxy-4-biphenylylcarbonyloxy]-4‘-biphenylcarboxylate with poly(ethylene oxide) with a degree of polymerization of 12 (12-4) was observed to exhibit a microphase-separated lamellar structure with nanoscale dimension and to melt into a layered smectic A mesophase. The complexes of 12-4 with 0.05−0.8 mol of LiCF3SO3 per ethylene oxide unit of a molecule were also prepared in order to investigate mesomorphic phase changes of 12-4 upon complexation. An abrupt mesophase change was observed for the entire range of salt concentrations. The complexes with 0.05−0.2 mol of LiCF3SO3 display an enantiotropic smectic A phase as their highest temperature mesophase. From the complex with 0.2 mol of LiCF3SO3, an enantiotropic cubic phase is induced and a smectic A phase disappears at the complex with 0.25 mol of LiCF3SO3, which displays a cubic mesophase only. The complex with 0.3 mol of LiCF3SO3 also exhibits an enantiotropic cubic phase; however, it exhibits a cylindrical micellar mesophase as its highest temperature mesophase, contrary to the complexes with up to 0.25 mol of LiCF3SO3. The complexes with 0.4−0.7 mol of LiCF3SO3exhibit only a cylindrical micellar mesophase, and the complex with higher salt concentration becomes amorphous. These results, characterized by a combination of differential scanning calorimetry, optical polarized microscopy, and X-ray scattering experiments, are discussed.