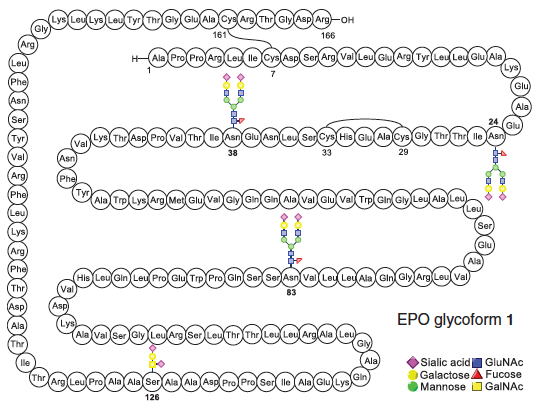
Wang, P.; Dong, S.; Shieh, J.-H.; Peguero, E.; Hendrickson, R.; Moore, M. A. S.; Danishefsky, S. J. Science 2013, 342, 1357–1360. DOI: 10.1126/science.1245095 Erythropoietin is a signaling ...
Posts by Category: OrganicChem
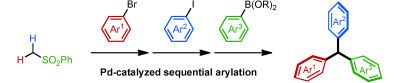
Modular Synthesis of Triarylmethanes through Palladium-Catalyzed Sequential Arylation of Methyl Phenyl Sulfone
Nambo, M.; Crudden, C. M. Angew. Chem. Int. Ed. 2013, Early view. DOI: 10.1002/anie.201307019 Triarylmethanes, which are valuable structures in materials, sensing and pharmaceuticals, have been ...
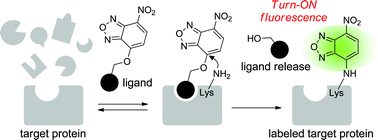
Turn-ON fluorescent affinity labeling using a small bifunctional O-nitrobenzoxadiazole unit
Yamaguchi, T.; Asanuma, M.; Nakanishi, S.;Saito Y.;,Okazaki, M.; Dodoab, K. Sodeoka, M. Chem. Sci., 2014, Advance Article DOI: 10.1039/C3SC52704B Affinity labeling has become a powerful tool to ...
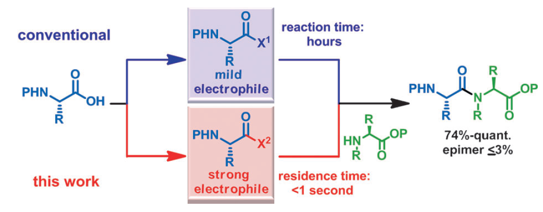
Efficient Amide Bond Formation through a Rapid and Strong Activation of Carboxylic Acids in a Microflow Reactor
Fuse, S.; Mifune, Y.; Takahashi, T. Angew. Chem. Int. Ed. 2013, Early View DOI: 10.1002/anie.201307987 The development of highly efficient amide bond forming methods which are devoid of side ...
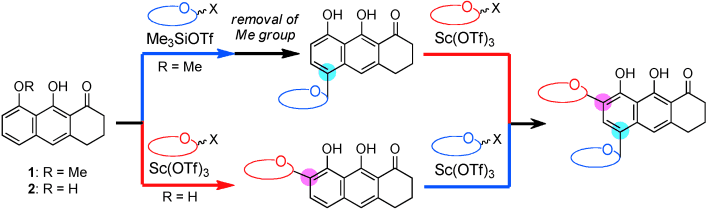
Synthesis of the Pluramycins 1: Two Designed Anthrones as Enabling Platforms for Flexible Bis-C-Glycosylation
Kitamura, K.; Ando, Y.; Matsumoto, T.; Suzuki, K. Angew. Chem. Int. Ed. 2013 Early View DOI: 10.1002/ange.201308016 Two effective tricyclic platforms are reported for the installation of the two ...
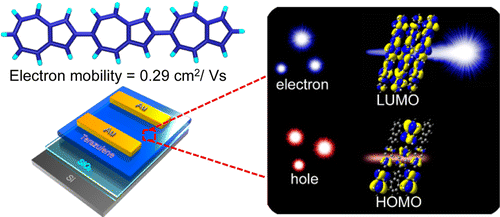
Terazulene: A High-Performance n‑Type Organic Field-Effect Transistor Based on Molecular Orbital Distribution Control
Yamaguchi, Y.; Ogawa, K.; Nakayama, K.-I.; Ohba, Y.; Katagiri, H. J Am Chem Soc 2013, ASAP. DOI: 10.1021/ja410696j We present herein a linear expanded π-conjugation system comprising azulene ...
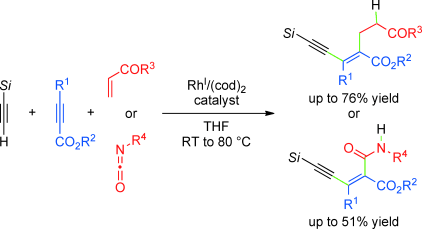
Rhodium-Catalyzed Three-Component Cross-Addition of Silylacetylenes, Alkynyl Esters, and Electron-Deficient Alkenes or Isocyanates
Hoshino, Y.; Shibata, Yu.; Tanaka, K.; Angew. Chem. Int. Ed. 2012, Early View. DOI: 10.1002/anie.201204646 ’Rh’oad crossing: A cationic RhI/(cod)2 complex catalyzes the chemo-, regio-, and ...
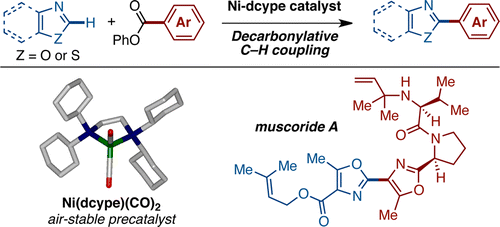
Decarbonylative C–H Coupling of Azoles and Aryl Esters: Unprecedented Nickel Catalysis and Application to the Synthesis of Muscoride A
Amaike, K.; Muto, K.; Yamaguchi, J.; Itami, K. J. Am. Chem. Soc., 2012, 134 , 13573–13576. DOI: 10.1021/ja306062c A nickel-catalyzed decarbonylative C–H biaryl coupling of azoles and aryl ...