Julie A. Peterson, Chamari Wijesooriya, Elizabeth J. Gehrmann, Kaitlyn M. Mahoney, Pratik P. Goswami, Toshia R. Albright, Aleem Syed, Andrew S. Dutton, Emily A. Smith, Arthur H. Winter. J. Am. Chem. Soc., 2018, ***, ****–*****.
DOI: 10.1021/jacs.8b04040
Abstract
Photocages are light-sensitive chemical protecting groups that provide external control over when, where, and how much of a biological substrate is activated in cells using targeted light irradiation. Regrettably, most popular photocages (e.g., o-nitrobenzyl groups) absorb cell-damaging ultraviolet wavelengths. A challenge with achieving longer wavelength bond-breaking photochemistry is that long-wavelength-absorbing chromophores have shorter excited-state lifetimes and diminished excited-state energies. However, here we report the synthesis of a family of BODIPY-derived photocages with tunable absorptions across the visible/near-infrared that release chemical cargo under irradiation. Derivatives with appended styryl groups feature absorptions above 700 nm, yielding photocages cleaved with the highest known wavelengths of light via a direct single-photon-release mechanism. Photorelease with red light is demonstrated in living HeLa cells, Drosophila S2 cells, and bovine GM07373 cells upon ∼5 min irradiation. No cytotoxicity is observed at 20 μM photocage concentration using the trypan blue exclusion assay. Improved B-alkylated derivatives feature improved quantum efficiencies of photorelease ∼20-fold larger, on par with the popular o-nitrobenzyl photocages (εΦ = 50–100 M–1 cm–1), but absorbing red/near-IR light in the biological window instead of UV light.
Introduction
Details
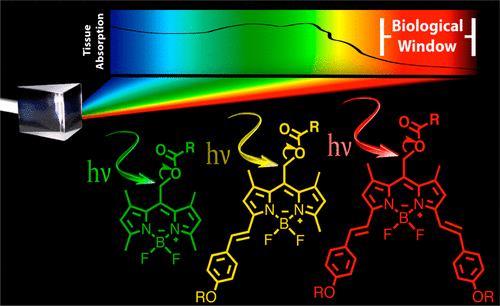
Figure 1 shows the substrates described in this study. Substrates 1 and 2 are from previous work, while 3–9 are from this work.
Figure 2 shows the absorbance (top) and emission (bottom) spectra of selected BODIPY photocages.
Figure 3 shows the fluorescence images of (A–D) HeLa cells incubated with 20 μM compound 10 irradiated with 635 nm light and (E–H) cells incubated with compound 10 with no irradiation as a function of time. (I) Average fluorescence intensity vs time of 10 incubated in HeLa cells (n = 10) with (i) and without (ii) 635 nm irradiation. (I) Increase in fluorescence of compound 10 in H2O with 5% BSA when irradiated.
Reference
[1]””
DOI:
[2]””
DOI: