Ya-Xuan Zhu, Hao-Ran Jia, Guang-Yu Pan, Nathan W. Ulrich, Zhan Chen, Fu-Gen Wu. J. Am. Chem. Soc. 2018, 140, 4062−4070.
DOI: 10.1021/jacs.7b13672
Abstract
Research on nanomedicines has rapidly prgressed in the past few years. However, due to the limited size of nuclear pores (9−12 nm), the nuclear membrane remains a difficult barrier to many nucleus-targeting agents. Here, we report the development of a general platform to effectively deliver chemical compounds such as drug molecules or nanomaterials into cell nuclei. This platform consists of a polyamine-containing polyhedral oligomeric silsesquioxane (POSS) unit, a hydrophilic polyethylene glycol (PEG) chain, and the photosensitizer rose bengal (RB), which can self- assemble into nanoparticles (denoted as PPR NPs). Confocal fluorescence imaging showed that PPR NPs mainly located in lysosomes after cellular internalization. After mild light irradiation, however, PPR NPs effectively disrupted lysosomal structures by singlet oxygen (1O2) oxidation and substantially accumulated on nuclear membranes, which enabled further disruption of the membrane integrity and promoted their final nuclear entry. Next, we selected two chemotherapeutic agents (10-hydroxycamptothecine and docetaxel) and a fluorescent dye (DiD) as payloads of PPR NPs and successfully demonstrated that this nanocarrier could efficiently deliver them into cell nuclei in a light-controlled manner. In addition to molecular compounds, we have also demonstrated that PPR NPs could facilitate the nuclear entry of nanomaterials, including Prussian blue NPs as well as gold nanorods. Compared to traditional strategies for nuclear delivery, this highly controllable nanoplatform avoids complicated modification of nucleus-targeting ligands and is generally applicable to both molecular compounds and nanomaterials.
Introduction
Nanomaterials have been extensively designed into effective drug delivery systems (DDSs). Although delivering drugs into cell nucleus that contains DNA sometimes can effectively induce cell death, they are usually entrapped in lysosomes after cellular internalization. To overcome the problem of efficient nuclear delivery, the designed platform must meet the following criteria: (1) it should have a high amount of cellular uptake, (2) it is able to accumulate in the perinuclear region, and (3) it is capable of facilitating the migration of diagnostic/therapeutic materials into the nucleus. [1]
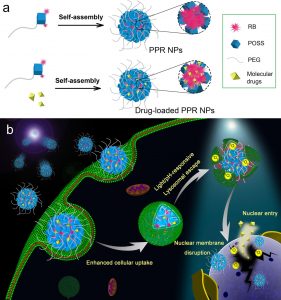
Herein, Zhan Chen group at University of Michigan and their coworkers introduced novel pH-responsive and light-triggerable nanocarrier platform for direct nuclear delivery via increased cell uptake, effective lysosomal escape, and augmented nuclear membrane accumulation and permeation. In their DDS design, a multiamine-containing polyhedral oligomeric silsesquioxane (POSS)[2] molecule is chemically conjugated with a PEG chain and several hydrophobic rose bengal (RB, a photosensitizer) molecules to form the final PEG−POSS−RB (abbreviated as PPR) conjugate.
Scheme 1. Schematics Illustrating (a) the Formation of PPR NPs and PPR/HCPT NPs and (b) the pH-Responsive and Light-Triggerable Nuclear Delivery Strategy Using PPR/HCPT NPs as an Example
Details
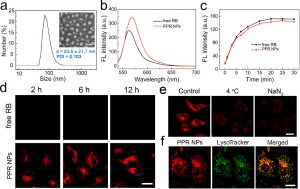
Figure 1 shows the characterization and cellular fate of PPR NPs. (a) DLS result of PPR NPs in a PBS solution. Inset: TEM image of PPR NPs (scale bar = 100 nm). (b) Fluorescence spectra of free RB and PPR NPs (3 μM RB in both samples) in PBS solutions. (c) 1O2 generation of free RB and PPR NPs in PBS solutions under 532 nm laser irradiation (8 mW/cm2) as reflected by the fluorescence intensity changes of SOSG. (d) Confocal fluorescence images of A549 cells after incubation with free RB or PPR NPs (3 μM RB in both samples) for different time periods. Scale bar = 25 μm. (e) Confocal fluorescence images of A549 cells after incubation with PPR NPs (3 μM RB) under different conditions as indicated. Scale bar = 10 μm. (f) Confocal fluorescence images of A549 cells stained by LysoTracker Green after incubation with PPR NPs (3 μM RB) for 2 h. Scale bar = 10 μm.
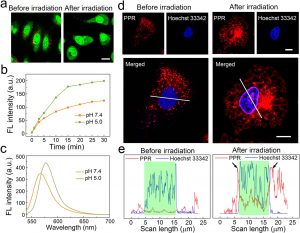
Figure 2 shows the results of lysosomal escape and nuclear membrane distribution of PPR NPs. (a) Observation of lysosomal disruption of A549 cells treated with PPR NPs before and after white light irradiation using AO staining. AO generates red fluorescence in acidic lysosomes and emits green fluorescence in cytosol and nucleus. Scale bar = 20 μm. (b) 1O2 generation of PPR NPs in the buffer solutions under laser irradiation (532 nm). (c) Fluorescence spectra of PPR NPs in the buffer solutions at pH 5.0 and 7.4. (d) Confocal fluorescence images of A549 cells stained by Hoechst 33342 after incubation with PPR NPs (3 μM RB) for 2 h before and after white light irradiation. Scale bars = 10 μm. (e) The corresponding fluorescence intensity profiles of the areas marked by white lines in the confocal images in part d. The green-shaded areas indicate the nuclear regions.
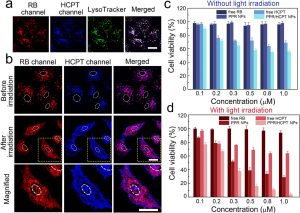
Figure 3 shows confocal fluorescence images and in vitro combination therapy of PPR/HCPT NPs. (a) Confocal fluorescence images of A549 cells stained by LysoTracker Green after incubation with PPR/HCPT NPs for 12 h. Scale bar = 10 μm. (b) Confocal fluorescence images of A549 cells treated with PPR/HCPT NPs before and after light irradiation. The images in the “Magnified” row come from the dotted squares in the “After irradiation” row. The white dotted circles represent the area of cell nuclei. Scale bar = 10 μm. (c) Viabilities of A549 cells after incubation with free RB, free HCPT, PPR NPs, or PPR/HCPT NPs at different concentrations of RB (0.1, 0.2, 0.3, 0.5, 0.8, and 1.0 μM) for 24 h. The cell viabilities were determined by CCK-8 assay. (d) Viabilities of A549 cells after incubation with free RB, free HCPT, PPR NPs, or PPR/HCPT NPs and white light irradiation (2 mW/cm2, 5 min). All these cells were then incubated for 24 h before CCK-8 assay.
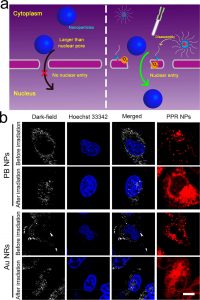
Figure 4(a) shows schematic diagram of light-promoted nuclear entry of nanoparticles larger than nuclear pores assisted by PPR NPs. Figure 4(b) The confocal images of A549 cells treated by PB NPs or Au NRs before and after light irradiation (2 mW/cm2, 3 min). The cell nuclei were stained blue by Hoechst 33342 using confocal fluorescence imaging, and the PB NPs and Au NRs were shown as white dots using dark- field imaging. Scale bar = 10 μm.
Reference
[1]”How successful is nuclear targeting by nanocarriers?”
Tammam, S. N.; Azzazy, H. M. E.; Lamprecht, A. J. Controlled Release 2016, 229, 140−153.
DOI: http://10.1016/j.jconrel.2016.03.022
[2]”Recent Developments in the Chemistry of Cubic Polyhedral Oligosilsesquioxanes”
Cordes, D. B.; Lickiss, P. D.; Rataboul, F. Chem. Rev. 2010, 110, 2709−2728.