- Generality
- Orthogonality to Other PGs
-
General Characteristics
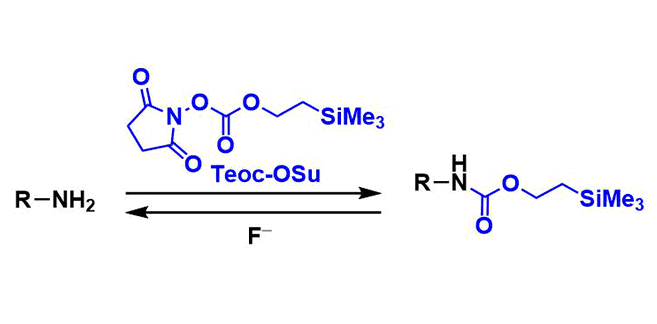
2-(Trimethylsilyl)ethoxycarbonyl (Teoc) group is one of commonly used carbamate protecting groups for amines. It is stable towards hydrolysis and other nucleophilic attacks, and tolerates most acidic and reductive conditions, including heterogeneous hydrogenation. Deprotection is most commonly performed by treatment with fluoride reagents such as TBAF.
-
General References
- Teoc-OSu: Shute, R. E.; Rich, D. H. Synthesis 1987, 346. DOI: 10.1055/s-1987-27939
- Teoc-NTShimizu, M.; Sodeoka, M. Org. Lett. 2007, 9, 5231. DOI: 10.1021/ol7024108
-
Reaction Mechanism
Protection
N-[2-(trimethylsilyl)ethoxycarbonyloxy]succinimide (Teoc-OSu) is the commonly used reagent to protect amines as Teoc carbamates. Bases such as pyridine or triethylamine are normally used. The Schotten-Baumann conditions, in which inorganic bases are used, are also viable for protecting amino acids.

Deprotection
Similar to other silyl-based protecting groups, Teoc can be deprotected using fluoride ion. The attack of fluoride to the silicon atom leads to β-elimination and decarboxylation, releasing the free amine along with ethylene, carbon dioxide, and Me3SiF.

-
Examples
The Sodeoka reagent (Teoc-NT) is useful to protect amines since the nitrotriazole byproduct is insoluble and can be removed by filtration alone.[1]

-
Experimental Procedure
-
Experimental Tips
-
References
Shimizu, M.; Sodeoka, M. Org. Lett. 2007, 9, 5231. DOI: 10.1021/ol7024108
-
Related Reactions
-
Related Books
-
External Links
Protecting Group (PG) (PDF)