Overall Score3.5
- Generality
- Reagent Availability
- Experimental User Friendliness
-
General Characteristics
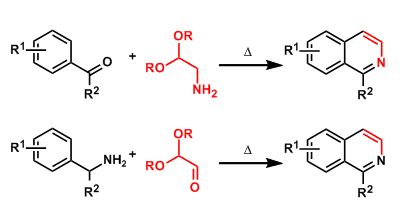
The condensation of benzaldehydes or arylketones with aminoacetaldehyde acetals gives isoquinolines.
Alternatively, the condensation of benzylamines with glyoxal hemiacetals also works for synthesizing the same products (the Schlittler-Muller modification).
-
General References
Pomeranz, C. Monatsh 1893, 14, 116.
Fritsch, P. Ber. 1893, 26, 419.
Schlittler, E.; Muller, J. Helv. Chim. Acta 1948, 31, 914, 1119.
-
Reaction Mechanism
-
Examples
-
Experimental Procedure
-
Experimental Tips
-
References
-
Related Reactions
Conrad-Limpach Quinoline Synthesis
Niementowski Quinoline/Quinazoline Synthesis
Bischler-Napieralski Isoquinoline Synthesis
Pfitzinger Quinoline Synthesis
Friedländer Quinoline Synthesis
Pictet-Gams Isoquinoline Synthesis
Doebner-von Miller quinoline synthesis
-
Related Books
-
External Links