Overall Score4.5
- Generality
- Importance in Conformational Studies
-
General Characteristics
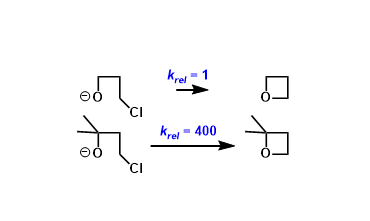
The effect of geminal substitution in linear molecules that promotes cyclization is referred to as the Thorpe-Ingold effect (aka. gem-dialkyl effect).
-
General References
- Beesley, R. M.; Ingold, C. K.; Thorpe, J. F. J. Chem. Soc. Trans. 1915, 107, 1080. DOI:10.1039/CT9150701080
- Jung, M. E.; Piizzi, G. Chem. Rev.2005, 105, 1735. DOI:10.1021/cr940337h
- Bachrach, S. M. J. Org. Chem.2008, 73, 2466. DOI: 10.1021/jo702665r
-
Reaction Mechanism
There are two main factors that are used to explain the Thorpe-Ingold effect.
- Compressed bond angle: Geminal alkyl substitution makes the bond angle between the two reacting groups narrower than 109.5˚(regular tetrahedral angle), which promotes cyclization (especially the formation of small rings).

- Decreased conformational freedom: The steric repulsion caused by the geminal substituents increases the probability of the molecule taking the conformation that favors cyclization.

-
Examples
The Thorpe-Ingold concept can also be used to rationalize equilibrium constants (thermodynamic phenomenon).

Cyclization of thioacetal-protected compounds tends to be much faster than that of unprotected ketone counterparts.

The “trimethyl lock” system, in which the lactonization is extremely fast due to the repulsions between three methyl groups, has been designed as a new tool for pro-drug and protecting group development.[1]

-
Experimental Tips
-
References
[1] Levine, M. N.; Raines, R. T. Chem. Sci. 2012, 3, 2412. DOI: 10.1039/C2SC20536J
-
Related Reactions
-
Related Books
-
External Links