Overall Score4
- Generality
- Reagent Availability
- Experimental User Friendliness
-
General Characteristics
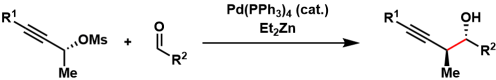
In the Marshall propargylation, propargyl mesylates and aldehydes are reductively coupled to form a new carbon-carbon bond. The transfer of chirality from the mesylates allows for reliable construction of contiguous stereogenic centers. Reagents such as diethylzinc and indium(I) iodide are used as reducing agents. This reaction has been demonstrated to be useful in large scale settings.
-
General References
- Marshall, J. A.; Adams, N. D. J. Org. Chem. 1998, 63, 3812. DOI: 10.1021/jo980623j
- Marshall, J. A.; Adams, N. D. J. Org. Chem. 1999, 64, 5201. DOI: 10.1021/jo9823083
- Marshall, J. A.; Grant, C. M. J. Org. Chem. 1999, 64, 696. DOI: 10.1021/jo982255p
- Marshall, J. A.; Chobanian, H. R.; Yanik, M. M. Org. Lett. 2001, 3, 3369. DOI: 10.1021/ol016605x
- Marshall, J. A.; Schaaf, G. M. J. Org. Chem. 2001, 66, 7825. DOI: 10.1021/jo015936k
<reviews>
- Marshall, J. A. J. Org. Chem. 2007, 72, 8153. DOI: 10.1021/jo070787c
- Ding, C.-H.; Hou, X.-L. Chem. Rev. 2011, 111, 1914. DOI: 10.1021/cr100284m
-
Reaction Mechanism
The mechanism involves generation of the chiral allenylzinc species involving redox cycle of the palladium catalyst. The allenyl zinc reacts with the aldehyde via a cyclic transition state with the transfer of chirality.

-
Examples
An example in the synthesis of lejjimalide B.[1]

Total synthesis of ushikulide A.[2]

-
Experimental Procedure
-
Experimental Tips
-
References
- Chen, Q.; Schweitzer, D.; Kane, J.; Davisson, V. J.; Helquist,P. J. Org. Chem. 2011, 76, 5157. DOI: 10.1021/jo200514m
- (a) Trost, B. M.; O’Boyle, B. M. J. Am. Chem. Soc. 2008, 130, 16190. (b) Trost, B. M.; O’Boyle, B. M.; Hund, D. J. Am. Chem. Soc. 2009, 131, 15061. DOI: 10.1021/ja906056v
-
Related Reactions
-
Related Books
-
External Links