- Generality
- Reagent Availability
- Experimental User Friendliness
-
General Characteristics
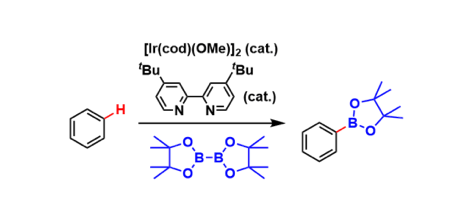
Iridium catalysts coordinated with electron-donating bidentate ligands promote direct C-H borylation of non-halogenated aromatic rings. Organoboronate compounds are, of course, valuable as reagents of various chemical transformations.
The regioselectivity of the C-H borylation is highly sensitive to steric properties of the substituents on the aromatic ring. Therefore, meta-selective functionalization is possible unlike directed ortho-metallations and the Friedel-Crafts type reactions.
See the Miyaura-Ishiyama borylation for the conditions in which halogenated aromatic substrates are borylated.
-
General References
- Cho, J. Y.; Tse, M. K.; Holmes, D.; Maleczka, R. E., Jr.; Smith, M. R. Science 2002, 295, 305. DOI:10.1126/science.1067074
- Ishiyama, T.; Takagi, J.; Ishida, K.; Miyaura, N.; Anastasi, N. R.; Hartwig, J. F. J. Am. Chem. Soc. 2002, 124, 390. DOI: 10.1021/ja0173019
- Ishiyama, T.; Takagi, J.; Hartwig, J. F.; Miyaura, N. Angew. Chem. Int. Ed. 2002, 41, 3056. [abstract]
- Ishiyama, T.; Nobuta, Y.; Hartwig, J. F.; Miyaura, N. Chem. Commun. 2003, 2924. DOI: 10.1039/B311103B
<reviews>
- Ishiyama, T.; Miyaura, N. Chem. Rec. 2004, 3, 271. DOI: 10.1002/tcr.10068
- Ishiyama, T.; Takagi, J.; Nobuta, Y.; Miyaura, N. Org. Synth. 2005, 82, 126. DOI: 10.15227/orgsyn.082.0126
- Mkhalid, I. A. I.; Barnard, J. H.; Marder, T. B.; Murphy, J. M.; Hartwig, J. F. Chem. Rev. 2010, 110,890. doi:10.1021/cr900206p
- Hartwig, J. F. Chem. Soc. Rev. 2011, 40, 1992. doi:10.1039/C0CS00156B
- Hartwig, J. F. Acc. Chem. Res. 2012, 45, 864. DOI: 10.1021/ar200206a
- Ros, A.; Femandez, R.; Lassaletta, J. M. Chem. Soc. Rev. 2014, 43, 3229. DOI: 10.1039/C3CS60418G
- Shinokubo, H. Proc. Jpn. Acad. Ser. B. 2014, 90, 1. doi:10.2183/pjab.90.1
- Yang, J. Org. Biomol. Chem. 2015, 13, 1930. DOI: 10.1039/C4OB02171A
-
Reaction Mechanism
The fac-tris(boryl)iridium(III) complex generated from the iridium precursor, the ligand, and the diboron reagent is considered as the active catalytic species. The C-H activation of the aromatic ring by oxidative addition occurs at the empty coordination site after dissociation of the olefin ligand. (Ref: J. Am. Chem. Soc. 1993, 115, 9329; J. Am. Chem. Soc. 2003, 125, 16114; J. Am. Chem. Soc. 2005, 127, 14263)

-
Examples
The C-H borylation is influenced most by the steric interactions. Ortho-borylation therefore tends to be slow. (The table is extracted from this webpage.)

Porphyrin can be borylated selectively at the β-position.[1]

C-H borylation as a key step in the total synthesis of complanadine A.[2]

Using hydrosilane as a directing group, the otherwise-difficult ortho-borylation can be done.[3]

The borylations of cyclopropanes[4] and other sp3 C-H bonds[5] have been made possible by recent advancements.

The catalytic system has been extended to the silyation of unactivated sp3 C-H bonds and the example shown below utilizes the neighboring hydroxyl group as a tether.[6] This is a new useful way to synthesize polyol compounds such as sugars, because the C-Si bonds can be converted into hydroxyl groups by the Tamao oxidation.[7]

An example of para-selective C-H borylation catalyzed by the iridium catalyst bearing a bulky diphosphine ligand.[8]

The rhodium-catalyzed terminal functionalization of unactivated alkanes.[9]

-
Experimental Procedure
-
Experimental Tips
-
References
- Hata, H.; Shinokubo, H.; Osuka, A. J. Am. Chem. Soc. 2005, 127, 8264. DOI: 10.1021/ja051073r
- Fischer, D.F.; Sarpong, R. J. Am. Chem. Soc. 2010, 132, 5926. DOI: 10.1021/ja101893b
- Boebel, T. A.; Hartwig, J. F. J. Am. Chem. Soc. 2008, 130, 7534.DOI:10.1021/ja8015878
- Liskey, C. W.; Hartwig, J. F. J. Am. Chem. Soc. 2013, 135, 3375. DOI: 10.1021/ja400103p
- (a) Liskey, C. W.; Hartwig, J. F. J. Am. Chem. Soc. 2012, 134, 12422. DOI: 10.1021/ja305596v (b) Li, W.; Liskey, C. W.; Hartwig, J. F. J. Am. Chem. Soc. 2014, 136, 8755. DOI: 10.1021/ja503676d (c) Miyamura, S.; Araki, M.; Suzuki, T.; Yamaguchi, J.; Itami, K. Angew. Chem. Int. Ed. 2015, 54, 846. DOI: 10.1002/anie.201409186
- (a) Simmon, E. M.; Hartwig, J. F. Nature 2012, 483, 70. doi:10.1038/nature10785 (b) Li, B.; Driess, M.; Hartwig, J. F. J. Am. Chem. Soc. 2014, 136, 6586. DOI: 10.1021/ja5026479
- Frihed, T. G.; Heuckendorff, M.; Pedersen, C. M.; Bols, M. Angew. Chem. Int. Ed. 2012, 51, 12285. DOI: 10.1002/anie.201206880
- Saito, Y.; Segawa, Y.; Itami, K. J. Am. Chem. Soc. 2015, 137, 5193. DOI: 10.1021/jacs.5b02052
- Chen, H.; Schlecht, S.; Semple, T. C.; Hartwig, J. F. Science 2000, 287, 1995. doi:10.1126/science.287.5460.1995
-
Related Reactions
- Catalytic C-H activation
- Brown Hydroboration
- Suzuki-Miyaura Cross Coupling
- Miyaura-Ishiyama Borylation
-
Related Books
-
External Links