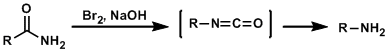- Generality
- Reagent Availability
- Experimental User Friendliness
-
General Characteristics
In the Hofmann rearrangement, primary amides rearrange into isocyanates upon treatment with halogens and bases and are ultimately converted into primary amines with one-carbon dehomologation.
The strongly basic conditions of the original procedure limit the synthetic practicality of the reaction. As alternatives, methods that use lead tetraacetate or hypervalent iodine reagents are known.
Carbamate-protected primary amines can be synthesized by adding appropriate alcohols.
-
General References
・Hofmann, A. W. Ber. 1881, 14, 2725.
・Wallis, E. S.; Lane, J. F. Org. React. 1949, 3, 267.
・Shioiri, T. Comprehensive Organic Synthesis 1991, 6, 800.
-
Reaction Mechanism

-
Examples
The synthesis of (-)-epibatidine[1]: The stereochemistry of the reactant is retained during the rearrangement.

An application to the synthesis of Tamiflu.[2]
-
Experimental Procedure
The procedure for a modified Hofmann rearrangement.[3]

To a 1-L, round-bottomed flask equipped with a stirring bar are added p-methoxybenzamide (10 g, 66 mmol), N-bromosuccinimide (NBS) (11.9 g, 66 mmol), 1,8-diazabicyclo[5.4.0]undec-7-ene (DBU) (22 mL, 150 mmol) and methanol (300 mL). The solution is heated at reflux for 15 min, at which point an additional aliquot of NBS (11.9 g, 66 mmol) is added slowly. The reaction is allowed to continue for another 30 min. Methanol is removed by rotary evaporation and the residue is dissolved in 500 mL of ethyl acetate (EtOAc). The EtOAc solution is washed with 6 N hydrochloric acid (HCl) (2 × 100 mL), 1 N sodium hydroxide (NaOH) (2 × 100 mL) and saturated sodium chloride (NaCl), and then dried over magnesium sulfate (MgSO4). The solvent is removed by rotary evaporation and the product, methyl N-(p-methoxyphenyl)carbamate, is purified by flash column chromatography [50 g of silica gel, EtOAc / hexane (1:1)] to give a pale yellow solid (11.1 g, 93%), which is further purified by recrystallization from 500 mL of hexane. Another 1.4 g of product (total 8.8 g, 73%) is obtained from the mother liquor by recrystallization from 100 mL of hexane.
-
Experimental Tips
-
References
[1] Evans, D. A.; Scheidt, K. A.; Downey, C. W. Org. Lett. 2001, 3, 3009. DOI: 10.1021/ol016420q
[2] Satoh, N.; Akiba, T.; Yokoshima, S.; Fukuyama, T. Angew. Chem. Int. Ed. 2007, 46, 5734. doi:10.1002/anie.200701754
[3] Keillor, J. W.; Huang, X. Org. Synth. 2002, 78, 234. [PDF]
-
Related Books