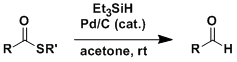- Generality
- Reagent Availability
- Experimental User Friendliness
-
General Characteristics
Thioesters can be converted into aldehydes directly without going through complete reduction to alcohols. By the Fukuyama procedure, the reduction can be effected under very mild conditions. Most functional groups are tolerated and substrate generality is also high.
This reaction is synthetically useful in that it is one of only few options available that allow for the direct conversion from the oxidation state of carboxylic acid to that of aldehyde.
-
General References
・Fukuyama, T. Lin, S.-C.; Li, L.J. Am. Chem. Soc. 1990, 112, 7050. DOI: 10.1021/ja00175a043
・Tokuyama, H.; Yokoshima, S.; Yamashita, T.; Lin, S.-C.; Li, L.; Fukuyama, T. Synthesis 2002, 1121. DOI: 10.1055/s-2002-31969
・福山透, 有機合成化学協会誌 2003, 61, 620.
・Fukuyama, T.; Tokuyama, H. Aldrichimica Acta 2004, 37, 85. [PDF]
-
Reaction Mechanism

-
Examples
Functional groups such as amides, esters, ketones, acetonides, silyl protecting groups, sulfides, and β-lactams are unreactive under the reaction conditions. Olefins do get reduced, but can be kept intact under the modified conditions using the Lindlar catalyst and excess amount of terminal alkene.[1]

The Fukuyama reduction is often used in natural product synthesis for its high functional group tolerance and mild reaction conditions.
Total synthesis of altohyrtin C (spongistatin 2)[2]: The reduction steps are used in effective and repetitive manner with the stereoselective Mukaiyama aldol reaction steps.

-
Experimental Procedure
Two-step synthesis of α-aminoaldehyde from α-amino acid.[3]
Step 1

Step 2

-
Experimental Tips
-
References
[1] Evans, D. A. et al. Angew. Chem., Int. Ed. Engl. 1997, 36, 2741. doi:10.1002/anie.199727411
[2] Evans, D. A. et al. Tetrahedron 1999, 55, 8671. doi:10.1016/S0040-4020(99)00438-X
[3] Tokuyama, H.; Yokoshima, S.; Yamashita, T.; Lin, S.-C.; Li, L.; Fukuyama, T. Synthesis 2002, 1121. DOI: 10.1055/s-2002-31969
-
Related Books