- Generality
- Reagent Availability
- Experimental User Friendliness
-
General Characteristics
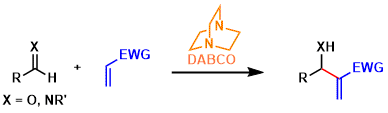
The Morita-Baylis-Hillman reaction is a type of carbon-carbon bond forming reactions between aldehydes/imines and electron deficient alkenes promoted by nucleophilic catalysts.
Cyclic tertiary amines like DABCO, DMAP, and DBU and phosphines are usually used as the nucleophilic catalyst.
The reaction tends to be slow under classical conditions. This remains as somewhat an unsolved problem today, since simple approaches such as adding Lewis acids and excessive heating cause problems like catalyst deactivation and alkene polymerization.
There have been many active researches in recent years on the development of the MBH reaction and its variants using asymmetric organocatalysis.
-
General References
・ Morita, K.; Suzuki, Z.; Hirose, H. Bull. Chem. Soc. Jpn. 1968, 41, 2815. DOI: 10.1246/bcsj.41.2815
・ Baylis, A. B.; Hillman, M. E. D. Chem. Abstr. 1972, 77, 34174q.
・ Drewes, S. E.; Roos, G. H. P. Tetrahedron 1988, 44, 4653. doi:10.1016/S0040-4020(01)86168-8
<Review>
・ Ciganek, E. Org. React. 1997, 51, 201. doi:10.1002/0471264180.or051.02
・ Basavaiah, D.; Rao, A. J.; Satyanarayana, T. Chem. Rev. 2003, 103, 811. DOI: 10.1021/cr010043d
・ Aggarwal, V. K.; Emme, I.; Fulford, S. Y. J. Org. Chem. 2003, 68, 692. doi:10.1021/jo026671s
・ Masson, G.; Housseman, C.; Zhu, J. Angew. Chem. Int. Ed. 2007, 46, 4614. doi:10.1002/anie.200604366
・ Declerck, V.; Martinez, J.; Lamaty, F. Chem. Rev. 2009, 109, 1. doi:10.1021/cr068057c
・ Basavaiah, D.; Reddy, B. S.; Badsara, S. S. Chem. Rev. 2010, 110, 5447. doi:10.1021/cr900291g
・ Basavaiah, D.; Veeraraghavaiah, G. Chem. Soc. Rev. 2012, 41, 68. doi:10.1039/C1CS15174F
・ Wei, Y.; Shi, M. Chem. Rev. 2013, 113, 6659. DOI: 10.1021/cr300192h
-
Reaction Mechanism
The reaction proceeds through the Michael addition of the catalyst, the aldol type addition, and the catalyst elimination. The proton transfer is presumed to be the rate determining step, therefore systems that could promote it tend to be more reactive. (Ref: Tetrahedron 1992, 48, 6371; Tetrahedron 1993, 49, 6931; J. Org. Chem. 2005, 70, 3980; Org. Lett.2005, 7, 147; Angew. Chem., Int. Ed. 2005, 44, 1706.)

-
Examples
The synthesis of salinosporamide A[1]: The intramolecular Morita-Baylis-Hillman reaction was used to construct the vicinal quaternary stereogenic centers.

The catalytic asymmetric MBH reaction using β-ICD.[2]

-
Experimental Procedure
-
Experimental Tips
-
References
[1] Rerddy, L. R.; Saravanan, P.; Corey, E. J.J. Am. Chem. Soc. 2004, 126, 6230. DOI: 10.1021/ja048613p
[2] Iwabuchi, Y.; Nakatani, M.; Yokoyama, N.; Hatakeyama, S. J. Am. Chem. Soc. 1999, 121, 10219. DOI: 10.1021/ja992655+
-
Related Books