- Generality
- Reagent Availability
- Experimental User Friendliness
-
General Characteristics
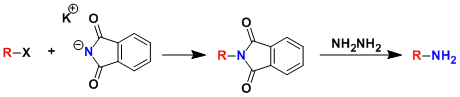
The synthesis of primary amines by way of alkylation of phthalimide is called the Gabriel amine synthesis. The use of DMF as the solvent generally increases the yield by accelerating the SN2 reaction. This method has been applied to the synthesis of amino acids as well.
The alkylated phthalimides (which can be thought of as protected amines) can be deprotected under various conditions such as treatment with hydrazine.
-
General References
・Gabriel, S. Ber. 1887, 20, 2224.
・Gibson, M. S.; Bradshaw, R. W. Angew. Chem. Int. Ed. Engl. 1968, 7, 919.
・Dietrich, B. et al. J. Am. Chem. Soc. 1981, 103, 1282.
・Mitsunobu, O. Comprehensive Organic Synthesis 1991, 6, 79.
・Sen, S. E.; Roach, S. L. Synthesis 1994, 756.
-
Reaction Mechanism
-
Examples
The Mitsunobu conditions are compatible for the alkylation step.[1]
-
Experimental Procedure
-
Experimental Tips
-
References
[1] Inoue, Y.; Taguchi, M.; Hashimoto, H. Synthesis 1986, 332.
-
Related Books

![gabrie3[1]](https://en.chem-station.com/wp-content/plugins/lazy-load/images/1x1.trans.gif)