- Generality
- Reagent Availability
- Experimental User Friendliness
-
General Characteristics
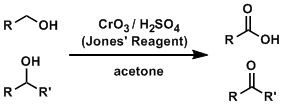
The Jones oxidation is one of the most fundamental reactions used to oxidize alcohol compounds. The reagents do not react with alkenes and alkynes and oxidize only alcohols. The reaction uses an aqueous sulfuric acid solution, therefore is inevitably unsuitable for compounds unstable under strongly acidic conditions.
The residue containing chromium compounds is highly toxic. Sufficient caution is required during handling and disposal.
-
General References
・ Bowden, K.; Heilbron, I. M.; Jones, E. R. H. J. Chem. Soc. 1946, 39.
・ Heilbron, I. M.; Jones, E. R. H.; Sondheimer, F. J. Chem. Soc. 1949, 604.
・ Ley, S. V.; Madin, A. Comprehensive Organic Synthesis 1991, 7, 253.
・ Luzzio, F. A. Org. React. 1998, 53, 1.
-
Reaction Mechanism
The reaction is carried out in an aqueous solution. The aldehyde intermediate is hydrated to form a gem-diol, which is oxidized further to give a carboxylic acid. (Which suggests that anhydrous condition is necessary to stop the oxidation at the aldehyde.)
-
Examples
-
Experimental Procedure
The preparation of the Jones reagent.
Dissolve chromium trioxide (25 g, 0.25 mol) in water (75 mL) in a 500 mL beaker and add concentrated sulfuric acid (25 mL) slowly with careful stirring and cooling in an ice-water bath. Keep the temperature of the solution between 0 and 5˚C. The concentration of the solution prepared by this procedure is 2.5 M.
-
Experimental Tips
-The addition of isopropanol is an easy way to quench the reaction.
-Chromium compounds (especially Cr(VI)) are highly toxic. One must take necessary caution when handling and disposing these compounds.
-
References
-
Related Books

![jones_oxi_2[1]](https://en.chem-station.com/wp-content/plugins/lazy-load/images/1x1.trans.gif)
online guide is very good but easy mefhod may should to understand mechanism