Overall Score4.5
- Generality
- Reagent Availability
- Experimental User Friendliness
-
General Characteristics
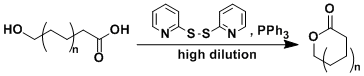
The macrolactonization reaction using 2,2’-dipyridyldisulfide and triphenylphosphine is known as the Corey-Nicolaou reaction. The cyclization step usually requires a high temperature. The addition of silver salts (e.g. AgBF4) for the activation of the pyridylthioester was later found to accelerate the reaction dramatically.
-
General References
・Corey, E. J.; Nicolaou, K. C. J. Am. Chem. Soc. 1974, 96, 5614. DOI: 10.1021/ja00824a073
・Corey, E. J.; Nicolaou, K .C.; Melvin, L. S., Jr. J. Am. Chem. Soc. 1975, 97, 653. DOI: 10.1021/ja00836a036
・Modification: Gerlach, H.; Thalmann, A. Helv. Chim. Acta 1974, 57, 2661.
-
Reaction Mechanism
-
Examples
The synthesis of zearalenone.[1]
-
Experimental Procedure
-
Experimental Tips
-
References
[1] Corey, E. J.; Nicolaou, K. C. J. Am. Chem. Soc. 1974, 96, 5614. DOI: 10.1021/ja00824a073
-
Related Books

![acid-a2[1]](https://en.chem-station.com/wp-content/plugins/lazy-load/images/1x1.trans.gif)