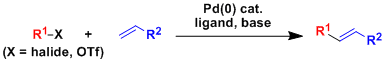- Generality
- Reagent Availability
- Experimental User Friendliness
-
General Characteristics
-The cross coupling reaction between an aryl/alkenyl halide and a terminal olefin in the presence of a Pd(0) catalyst to produce a substituted olefin is called the Mizoroki-Heck reaction.
-The reaction is highly functional group selective and high yielding.
-The reaction is especially effective in obtaining products in which the double bond does not migrate. When the substrate is an ally alcohol, olefin migration leads to the formation of a carbonyl product.
-For alkenes substituted with electron-withdrawing groups such as aryl or alkoxycarbonyl, coupling reactions take place at the unsubstituted carbon. On the other hand, the regioselectivity of alkenes with electron-donating groups such as alkoxyether is more difficult to control.
-Intramolecular Heck reaction (along with the Diels-Alder reaction) is one of the few methods capable of synthesizing fused ring systems containing quaternary stereogenic carbon(s).
-
General References
・Mizoroki, T.; Mori, K.; Ozaki, A. Bull. Chem. Soc. Jpn. 1971, 44, 581.
・Heck, R. F.; Nolley, Jr., J. P. J. Org. Chem. 1972, 37, 2320. DOI:10.1021/jo00979a024
・Heck, R. F. Org. React. 1982, 27, 345.
・de Meijere, A.; Meyer, F. E. Jr.; Angew. Chem. Int. Ed. Engl. 1994, 33, 2379.[Abstract]
・足森厚之, Overman, L. E. 有機合成化学協会誌 2000, 58, 718.
・Beletskaya, I. P.; Cheprakov, A. B. Chem. Rev. 2000, 100, 3009. DOI: 10.1021/cr9903048
・Dounay, A. B.; Overman, L. E. Chem. Rev. 2003, 103, 2945. DOI: 10.1021/cr020039h
・Link, J. T. Org. React. 2003, 103, 2945.
-
Reaction Mechanism
Both migratory insertion and β-hydride elimination occur in syn orientation to the palladium. This is important to understand the stereochemistry of the Heck reaction (especially in the cases involving the synthesis of fused ring systems).

-
Examples
The synthesis of arenastatin A.[1]

The Heck reaction using arylamines.

Trapping the π-allylpalladium intermediate[2]: The amide nitrogen attacks from the opposite face of the palladium.

Domino Heck reaction in the context of scopadulcic acid A synthesis[3]: The absence of β-hydrides to eliminate leads to the insertion into the second olefin, leading to the formation of the multicyclic product.

The synthesis of epitazocine by asymmetric Heck reaction[4]: Asymmetric Heck reaction is possible using chiral bidentate phosphine ligands. Cationic palladium is important for achieving high selectivity. Either triflate substrates or the addition of a silver salt for halide substrates is needed.

The stable palladacycle complex prepared from palladium acetate and P(o-Tol)3 can improve the catalytic efficiency up to about a million TON.

-
Experimental Procedure
The Mizoroki-Heck reaction between alkenyl bromide and methyl acrylate.[5]

-
Experimental Tips
-
References
[1] Georg, G. I.. et al. J. Org. Chem, 2000, 65, 7792. DOI: 10.1021/jo000767+
[2] Waterlot, C; Couturier, D. et.al. Tetrahedron Lett. 2000, 41, 317. doi:10.1016/S0040-4039(99)02082-1
[3] Fox, M. E.; Li, C; Marino, J. P.; Overman, L. E. J. Am. Chem. Soc. 1999, 121, 5467. DOI: 10.1021/ja990404v
[4] Takemoto, T.; Sodeoka, M.; Sasai, H.; Shibasaki, M. J. Am. Chem. Soc. 1993, 115, 8477. DOI: 10.1021/ja00071a079
[5] Dieck, H. A.; Heck, R. F. J. Org. Chem. 1975, 40, 1083. DOI: 10.1021/jo00896a020
-
Related Books