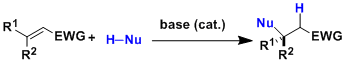- Generality
- Reagent Availability
- Experimental User Friendliness
-
General Characteristics
The Michael reaction originally meant the 1,4-addition (conjugate addition) of simple anionic carbons to the electron poor double bonds conjugated to carbonyl groups. Today’s expanded definition includes alkenes and alkynes adjacent to electron-withdrawing groups other than carbonyls as the acceptors and organometallic (such as RLi, RMgX, R2CuLi), amino, and alkoxide nucleophiles as the donors.
Various asymmetric versions of the reaction have been developed.
-
General References
・Michael, A. J. Prakt. Chem. 1887, 35, 349. doi:10.1002/prac.18870350136
・Michael, A. J. Prakt. Chem. 1894, 49, 20. doi:10.1002/prac.18940490103
・Bergmann, E. D.; Corett, R. J. Org. Chem. 1958, 23, 1507. DOI: 10.1021/jo01104a028
・Bergmann, E. D.; Ginsburg, D.; Pappo, R. Org. React. 1959, 10, 179.
・Lacey, R. N. J. Chem. Soc. 1960, 1625.
・Jung, M E. Comp. Org. Syn. 1991, 4, 1.
・Lee, V. J. Comp. Org. Syn. 1991, 4, 69, 139.
・Kozlowski, J. A. Comp. Org. Syn. 1991, 4, 169.
・d’Angelo J. et al. Tetrahedron: Asymmetry 1992, 3, 459. doi:10.1016/S0957-4166(00)80251-7
・Leonard, J. et al. Eur. J. Org. Chem. 1998, 2051. [abstract]
-
Reaction Mechanism
The activated proton of malonate ester is easily deprotonated. The enolate of the Michael adduct is strongly basic, thus deprotonates another malonate. Since this process is almost irreversible, the ratio of the nucleophile to the electrophile can be 1:1 to obtain high yield unlike aldol reactions. The base can be used in substoichiometric amounts.

-
Examples
The construction of the CD ring of steroid skeleton based on the Michael addition of the organocopper reagent.[1]

Shibasaki’s La-linked-BINOL complex is a highly active asymmetric Michael addition catalyst, which is also stable enough to be stored in open air without loss of activity.[2]

Lewis acid-catalyzed Michael addition of sily enol ether (see the Mukaiyama aldol reaction).[3]

-
Experimental Procedure
-
Experimental Tips
-
References
[1] 大学院講義有機化学II P.243
[2] Kim, Y. S. ; Matsunaga, S.; Das, J.; Sekine, A.; Ohshima, T.; Shibasaki, M. J. Am. Chem. Soc. 2000, 122, 6506. DOI: 10.1021/ja001036u
[3] (a) Narasaka, K.; Soai, K.; Aikawa, Y.; Mukaiyama, T. Bull. Chem. Soc. Jpn. 1976, 49, 779. doi:10.1246/bcsj.49.779 (b)Mukaiyama, T.; Kobayashi, S. Heterocycles 1987, 25, 245.
-
Related Books