-
Characteristics
N-hydroxyphthalimide (NHPI) and related compounds have been revealed to be efficient organocatalysts for free-radical processes and have found ample application in promoting the aerobic oxidation of a wide range of organic substrates. NHPI is a cheap, nontoxic catalyst easily prepared by thereaction of phthalic anhydride and hydroxylamine.
When combined with different co-catalysts, they are activated to the corresponding N-oxyl radical species (i.e. phthalimido-N -oxyl (PINO) radical) and become able to promote radical chains, involving molecular oxygen, directly or indirectly. Most of the examples reported in the literature describe the use of these N-hydroxy derivatives in the presence of transition-metal complexes.
NHPI and its combination with tansition-matal catalysts have recently been introduced as an effective system for C– H activation by hydrogen abstraction.
-
Literature reference
・Ishii, Y.; Nakayama, K.; Takeno, M.; Sakaguchi, S.; Iwahama, T.; Nishiyama, Y. J. Org. Chem. 1995,60, 3934. DOI: 10.1021/jo00118a002
<review>
・Ishii, Y.; Sakaguchi, S.; Iwahama, T. Adv. Synth. Catal. 2001, 343, 393. [abstract]
・Recupero, F.; Punta, C. Chem. Rev. 2007, 107, 3800. DOI: 10.1021/cr040170k
・Melone, L.; Punta, C. Beil. J. Org. Chem. 2013, 9, 1296. DOI: 10.3762/bjoc.9.146
-
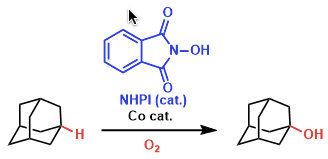
Reaction mechanism
Nowadays, the selective oxidation of alkanes with high conversion is one of the most challenging problems remaining to be solved. Alkanes were successfully oxidized with O2 or air to alcohols by the combined catalytic system of NHPI and a transition metal such as Co or Mn as shown below.

-
Example of reactions
Inoue reported metal-free direct C(sp3 ) H fluorination using a catalytic system consisting of N ,N –dihydroxypyromellitimide (NDHPI), which is one of NHPI derivatives and Selectfluor.[1]
Inoue and coworkers also reported an effi cient method for direct intermolecular conversion of C(sp3 )− H bonds to C(sp3 )− N bonds by employing a reagent system composed of catalytic N hydroxyphthalimide (NHPI) and stoichiometric dialkyl azodicarboxylate.[2]
-
Bibliography
[1] “Metal-Free Fluorination of C(sp3)–H Bonds Using a Catalytic N-Oxyl Radical”
Amaoka, Y.; Nagatomo, M.; Inoue, M. Org. Lett. 2013, 15, 2160. DOI: 10.1021/ol4006757
A direct conversion of C(sp3)–H bonds to C(sp3)–F bonds has been developed. In this process, a catalytic N-oxyl radical generated from N,N-dihydroxypyromellitimide abstracts hydrogen from the C(sp3)–H bond and Selectfluor acts to trap the resulting carbon radical to form the C(sp3)–F bond. This simple metal-free protocol enables the chemoselective introduction of a fluorine atom into various aromatic and aliphatic compounds and serves as a powerful tool for the efficient synthesis of fluorinated molecules.
[2] “Radical Amination of C(sp3)–H Bonds Using N-Hydroxyphthalimide and Dialkyl Azodicarboxylate”
Amaoka, Y.; Kamijo, S.; Hoshikawa, T.; Inoue, M. J. Org. Chem. 2012, 77, 9959. DOI:10.1021/jo301840e
A direct conversion of C(sp3)–H bonds to C(sp3)–N bonds has been achieved by utilizing catalytic N-hydroxyphthalimide (NHPI) and stoichiometric dialkyl azodicarboxylate. NHPI functions as a precursor of the electron-deficient phthalimide N-oxyl radical (PINO) to abstract hydrogens, and dialkyl azodicarboxylate acts as a trapping agent of the resultant carbon radical to generate the hydrazine derivatives. This C–H amination proceeds in a highly chemoselective manner with a wide applicability to functionalize benzylic, propargylic, and aliphatic C–H bonds. Furthermore, the obtained hydrazine compounds were readily converted to the corresponding carbamates or amines. Hence, the present protocol for direct introduction of the nitrogen functionality serves as a powerful tool for efficient construction of nitrogen-substituted natural products and pharmaceuticals.
-
Related Books