A, Wrobel.; T, Johnstone.; A, Liang.; S, Lippard.; Pablo Rivera-Fuentes. J. Am. Chem. Soc. 2014, 136, 4697
DOI: 10.1021/ja500315x
The first near-infrared fluorescent turn-on sensor for the detection of nitroxyl (HNO), the one-electron reduced form of nitric oxide (NO), is reported. The new copper-based probe, CuDHX1, contains a dihydroxanthene (DHX) fluorophore and a cyclam derivative as a Cu(II) binding site. Upon reaction with HNO, CuDHX1 displays a five-fold fluorescence turn-on in cuvettes and is selective for HNO over thiols and reactive nitrogen and oxygen species. CuDHX1 can detect exogenously applied HNO in live mammalian cells and in conjunction with the zinc-specific, green-fluorescent sensor ZP1 can perform multicolor/multianalyte microscopic imaging. These studies reveal that HNO treatment elicits an increase in the concentration of intracellular mobile zinc.
Introduction
HNO molecule not only play very important role in living organism but also is excretion molecule that results from variety of biological activities. Therefore, to visualize their pinpoint localization enables researcher to have a deeper understanding of Endogenous bioactivity. Although some dyes that selectively detect the presence of NO is now available from chemical companies, the advent of new near-infrared emission dye with high selectivity is highly is anticipated.
Working mechanism
Incorporating Cu atom with nitrogen chelating agent is widely used strategy to prepare HNO fluorescent sensitizer like CuBOT1 and CuCOT1.(1)(2) In the chelated state, the photo-induced electron transfer (PET) from the dye’s singlet excited state to the bound Cu2+ ion occurs, resulting the fluorescence OFF-state. Once the Cu2+ are reduced to Cu+ by NO molecule, the Cu atoms take apart and leave the dyes in fluorescence OFF-state. By applying same strategy, the group achieved the first near-infrared fluorescent turn-on sensor for the detection of HNO as shown below
Molecular design
(1) Commercial available IR-780 was used as starting material.
(2) Target compound DHX1 with long conjugated system (highlighted in red) was obtained.
(3) Cyclam (1,4,8,11-tetraazacyclotetradecane) was chosen as copper chelate side.(3)
Selectivity and spectrum
Various oxidant reagents including were tested for CuDHX1 to see the selectivity against HNO. As can be seen below, CuDHX1 was the most sensitive to HNO. The increase in fluorescence intensity and corresponding physical data are summarized below.
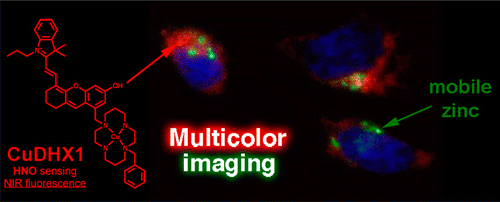
Multicolor imaging
To confirm its adaptability and functionality for practical uses, CuDHX1 (red emission) in combination with Hoechst 33528 dye (Blue DNA stainer) and ZP1 (green zinc sensitizer) was used to stain Hela cell. Addition of appropriate reagents for fluorescence activation revealed that CuDHX1 worked without any interference in other dyes. As shown in the last picture, three dyes functioned independently and could be detected through each channel.
Reference
[1]”Direct Detection of Nitroxyl in Aqueous Solution Using a Tripodal Copper(II) BODIPY Complex”
Rosenthal, J.; Lippard, S. J. J. Am. Chem. Soc. 2010, 132, 5536. DOI: 10.1021/ja909148v
The synthesis, photophysical properties, and a biological application of BODIPY-triazole 1 (BOT1) are described. BOT1 juxtaposes a BODIPY fluorophore with a tripodal ligand platform via a triazole bridge. The triazole linker is afforded by azide−alkyne “click” chemistry and comprises the third arm of the tripodal architecture. BOT1 binds Cu2+ in aqueous solution to form a CuII[BOT1] complex in which emission from the BODIPY moiety is efficiently quenched. This complex exhibits a prompt turn-on response when exposed to nitroxyl, and this emission response is specific to HNO over other reactive nitrogen and oxygen species. Notably, CuII[BOT1] does not fluoresce in the presence of nitric oxide, making this system the first discrete molecular probe capable of detecting HNO over NO under physiologically relevant conditions. Fluorescence microscopy experiments establish that CuII[BOT1] is membrane-permeable and can successfully signal the presence of HNO in live cells.
[2]”Visualization of Nitroxyl in Living Cells by a Chelated Copper(II) Coumarin Complex”
Zhou, Y.; Liu, K.; Li, J.-Y.; Fang, Y.; Zhao, T.-C.; Yao, C. Org. Lett. 2011, 13 1290. DOI: 10.1021/ol103077q
The coumarin-based probe Cu(II)-COT1 was successfully developed for the detection of HNO on the basis of the reduction reaction. In addition, highly selective “turn on” type fluorogenic behavior upon the addition of Angeli’s salt (Na2N2O3) was also applied to bioimaging in A375 cells.
[3]”Development of a Highly Selective Fluorescence Probe for Hydrogen Sulfide”
Sasakura, K.; Hanaoka, K.; Shibuya, N.; Mikami, Y.; Kimura, Y.; Komatsu, T.; Ueno, T.; Terai, T.; Kimura, H.; Nagano, T . J. Am. Chem. Soc. 2011, 133, 18003. DOI: 10.1021/ja207851s
Hydrogen sulfide (H2S) has recently been identified as a biological response modifier. Here, we report the design and synthesis of a novel fluorescence probe for H2S, HSip-1, utilizing azamacrocyclic copper(II) ion complex chemistry to control the fluorescence. HSip-1 showed high selectivity and high sensitivity for H2S, and its potential for biological applications was confirmed by employing it for fluorescence imaging of H2S in live cells.
[4]”Nitric Oxide Destroys Zinc-Sulfur Clusters Inducing Zinc Release from Metallothionein and Inhibition of the Zinc Finger-Type Yeast Transcription Activator LAC9″
Kröncke, K. D.; Fehsel, K.; Schmidt, T.; Zenke, F. T.; Dasting, I.; Wesener, J. R.; Bettermann, H.; Breunig, K. D.; Kolb-Bachofen, V. Biochem. Biophys. Res. Commun. 1994, 200, 1105. DOI: 10.1006/bbrc.1994.1564