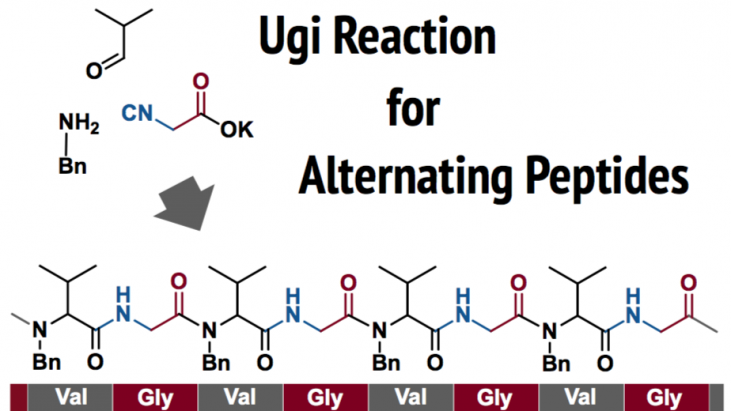
Koyama and Gudeangadi at Toyama Prefectural University developed an Ugi-reaction-based synthetic method of producing alternating peptides.
“One-pot synthesis of alternating peptides exploiting a new polymerization technique based on Ugi’s 4CC reaction”
Koyama, Y.*; Gudeangadi, P. G. Chem. Commun. 2017, 53, 3846–3849. DOI:10.1039/C6CC09379E
Introduced by H.O.
Issue
Polypeptides with a repeating sequence represent a promising candidate for innovative structural materials. Whereas numerous synthetic methods of polypeptides have been developed, the alternating copolymerization technique for polypeptide has not been sufficiently developed. Polycondensation of dipeptide as a monomer can afford the corresponding alternating peptide [1], but the preparation of dipeptide monomers is often troublesome.
To address this issue, Koyama and Gudeangadi at Toyama Prefectural University have developed a new, one-pot synthetic technique of alternating peptides. The technique is a polymerization analogous to Ugi’s 4-component reaction for the synthesis of dipeptides.
Approach
A typical Ugi’s four component reaction (4CR) gives dipeptide from a stoichiometric mixture of amine (R1NH2), aldehyde (R2CHO, or ketone), isonitrile (R3NC), and carboxylic acid (R4COOH)[2]. Their idea for the new polymerization technique is to connect the R3 and R4 groups. The reaction of imines with the ambident molecule bearing both isonitrile and carboxylic acid should result in polymerization to give the alternating peptides. Whereas polymer synthesis via Ugi reaction is known [3], the synthesis of alternating peptides has remained to be achieved.

Result
They chose potassium isocyanoacetate as a reactant, as this compound has sufficient stability as compared with the carboxylic acid analogue [4]. Reactive ambident molecule bearing both isonitrile and carboxylic acid functionalities was generated in-situ by the addition of a stoichiometric amount of TfOH.
As a result of optimization of reaction conditions, they achieved the one-pot synthesis of alternating peptides in 2-propanol. The present method is a good example of “click polymerization techniques”.

Points and Questions
- No polymerization catalyst is requested.
- Imines can be in situ generated from amines and aldehydes.
- Molecular weight was determined by DOSY (NMR technique).
- How to extend this reaction to the chiral version?
- The potassium α-isocyanoacetate was synthesized from the ester and KOH.
Author Information
PI Prof. Yasuhito Koyama (Toyama Pref. Univ.)
Career Summary
2005 Ph.D. at Tohoku Univ.
2007 Assist. Prof. at TiTech.
2013 Assoc. Prof. at Hokkaido Univ.
2016 Assoc. Prof. at Toyama Pref. Univ.
Cited from Koyama group site.
References
- Mandelkern, L.; Mattice, W. L. Biochemistry, 1971, 10, 1926–1933. DOI: 10.1021/bi00786a029
- Ugi, I. Angew. Chem., Int. Ed. Engl. 1962, 1, 8–21. DOI:10.1002/anie.196200081
- Rudick, J. G. J. Polym. Sci., Part A: Polym. Chem. 2013, 51, 3985–3985.
- Bonne, D.; Dekhane, M.; Zhu, J. Org. Lett. 2004, 6, 4771–4774. DOI: 10.1021/ol0479388