H, Masai.; J, Terao.; S, Seki.; S, Nakashima.; M, Kiguchi.; K, Okoshi.; T, Fujihara.; Y, Tsuji
DOI: 10.1021/ja411665k
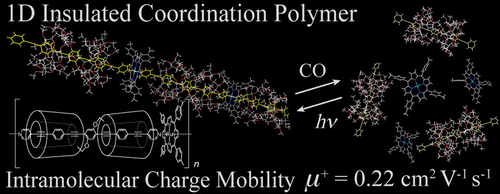
We report, herein, the design, synthesis, and properties of new materials directed toward molecular electronics. A transition metal-containing insulated molecular wire was synthesized through the coordination polymerization of a Ru(II) porphyrin with an insulated bridging ligand of well-defined structure. The wire displayed not only high linearity and rigidity, but also high intramolecular charge mobility. Owing to the unique properties of the coordination bond, the interconversion between the monomer and polymer states was realized under a carbon monoxide atmosphere or UV irradiation. The results demonstrated a high potential of the metal- containing insulated molecular wire for applications in molecular electronics.
Terao group, Kyoto University reported metal- containing insulated molecular wire for applications in molecular electronics to J. Am. Chem. Soc.
<Introduction>
Since its first introduction, insulated molecular wires (IMWs) suggested the possibility that, may be someday in the future, it can replace the present widely used wire. Typical weirs have a shared structure, that is, metal with high conductivity is wrapped with insulating polymer sheet. This design minimizes the loss of electricity and hence enabled its long-distance transportation.
<Molecular design>
To fully take advantage of the structural benefits based on the previously reported article[1], authors established wire-like system that is composed of three constituents.

(1) The electrical conductive core is made of long pi-system where pyridine is attached to both sides of oligo(phenylene ethynylene) (OPE).
(2) Donut shaped α-cyclodextrin (α-CD) is adopted as insulator sheet because of their donuts-shaped architecture and insulation property.
(3) Rh(TTP)Cl (TTP: tetratolylporphyrin) was perpendicularly attached to each side of the wire to ensure that the entire wire be liner and stiffened.
<Characterization>
In addition to the synthesis of nano-wires, detail photo-physical measurements like,conductivity transient observation, polarized optical analysis were also carried out. These experiment revealed that the nano-wires were in liquid crystal form under ambient conditions and had high conductivity.
<Reversible behavior>
The most surprising properties of this system is its external stimuli-responsive behavior. The long nano-wire can be cut into pieces and spontaneously self-healed on alternative exposing CO gas and UV-irradiation.[2] This process can be realized repeatedly and the detail mechanism and according fluorescence spectrum are shown below.
-
References
[1] “Insulated Molecular Wires”
Frampton, M. J.; Anderson, H. L. Angew. Chem., Int. Ed. 2007, 46, 1028. DOI: 10.1002/anie.200601780
An astonishing assortment of structures have been described as “insulated molecular wires” (IMWs), thus illustrating the diversity of approaches to molecular-scale insulation. These systems demonstrate the scope of encapsulation in the molecular engineering of optoelectronic materials and organic semiconductors. This Review surveys the synthesis and structural characterization of IMWs, and highlights emerging structure–property relationships to determine how insulation can enhance the behavior of a molecular wire. We focus mainly on three IMW architectures: polyrotaxanes, polymer-wrapped π systems, and dendronized polymers, and compare the properties of these systems with those of conjugated polymers threaded through mesoporous frameworks and zeolites. Encapsulation of molecular wires can enhance properties as diverse as luminescence, electrical transport, and chemical stability, which points to applications in electroluminescent displays, sensors, and the photochemical generation of hydrogen.
[2] “Reversible intramolecular electron transfer within a ruthenium(III) porphyrin-ruthenium(II) porphyrin π-cation radical system induced by changes in axial ligation ”
Barley, M. H.; Dolphin, D.; James, B. R. J. Chem. Soc., Chem. Commun. 1984, 1499. DOI: 10.1039/C39840001499
Reaction of the octaethylporphyrinatobis(triphenylarsine)ruthenium(III) cation with CO generates the carbonyl(triphenylarsine)ruthenium(II) porphyrin π-cation radical species viaan intramolecular electron transfer; the process is quantitatively reversible.
-
Related Books