El-Hellanid, A.; Lavallo, V. Angew. Chem. Int. Ed. 53, 2014. 4489-4493.
DOI: 10.1002/anie.201402445
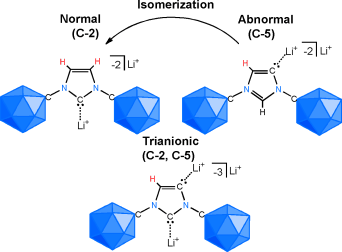
Fusion of two families of unusual carbon-containing molecules that readily disregard the tendency of carbon to form four chemical bonds, namely N-heterocyclic carbenes (NHCs) and carborane anions. Deprotonation of an anionic imidazolium salt with lithium diisopropylamide at room temperature leads to a mixture of lithium complexes of C-2 and C-5 dianionic NHC constitutional isomers as well as a trianionic (C-2, C-5) adduct. Judicious choice of the base and reaction conditions allows the selective formation of all three stable polyanionic carbenes. In solution, the so-called abnormal C-5 NHC lithium complex slowly isomerizes to the normal C-2 NHC, and the process can be proton-catalyzed by the addition of the anionic imidazolium salt. These results indicate that the combination of two unusual forms of carbon atoms can lead to unexpected chemical behavior, and that this strategy paves the way for the development of a broad new generation of NHC ligands for catalysis.
NHCs are not only recognized as the most useful ligands for transition metal catalysts, but known as the organo-catalysts with their own unique property. Note that NHCs conventionally used for applications are electronically neutral. Although their nature as both ligands and organocatalysts is already excellent, if they can carry charges, thus, such poly anionic NHCs will open the door for the further advance in catalysis fields.
In this report, Vince group shows that NHC can formally possess mono-, di-, and tri-anion in the molecule by appending anionic carborane as the substituents on N atoms in the hetero cycle skeleton. Interestingly, these new NHCs contain both low valent (carbene carbon) and hyper valent (carborane carbon) carbons in a molecule. By using a similar type of anionic ligand where an perchloro- anionic carborane is substituted on the P atom, previously, Vince group has already demonstrated the superior ligand’s feature to enhance the catalytic ability of transition metal complex for hydroamination reaction.[1]
Since a variety of carboranes are reported thus far, synthetically accessible, and in fact some of them are nowadays commercially available, new class of multi anionic ligands featuring various carboranes will be approachable. This pretty unique and original strategy will pave the way for the development of a broad new generation of ligands, as Vince et al. mentioned.
-
References
[1] “Perhalogenated Carba-closo-dodecaborate Anions as Ligand Substituents: Applications in Gold Catalysis
Lavallo, V.; Wright II, J. H.; Tham, F. S.; Quinlivan. S. Angew. Chem. Int. Ed. 2013, 52, 3172 – 3176. DOI: 10.1002/anie.201209107
The single-component zwitterionic gold catalyst formed by the coordination of a phosphine ligand bearing an inert and noncoordinating carborane substituent, CB11Cl11−, to an AuI ion exhibited the highest reported activity for the hydroamination of alkynes with amines (see scheme). The highest turnover number observed for hydroamination with this catalyst exceeded a stunning 95 000.
- Related Links
Lavallo Lab home Page