Ori Green, Samer Gnaim, Rachel Blau, Anat Eldar-Boock, Ronit Satchi-Fainaro, Doron Shabat. J. Am. Chem. Soc., 2017, 139, 13243–13248.
DOI: 10.1021/jacs.7b08446
ABSTRACT
Chemiluminescent luminophores are considered as one of the most sensitive families of probes for detection and imaging applications. Due to their high signal-to-noise ratios, luminophores with near-infrared (NIR) emission are particularly important for in vivo use. In addition, light with such long wavelength has significantly greater capability for penetration through organic tissue. So far, only a few reports have described the use of chemiluminescence systems for in vivo imaging. Such systems are always based on an energy-transfer process from a chemiluminescent precursor to a nearby emissive fluorescent dye. Here, we describe the development of the first chemiluminescent luminophores with a direct mode of NIR light emission that are suitable for use under physiological conditions. Our strategy is based on incorporation of a substituent with an extended π-electron system on the excited species obtained during the chemiexcitation pathway of Schaap’s adamantylidene-dioxetane probe. In this manner, we designed and synthesized two new luminophores with direct light emission wavelength in the NIR region. Masking of the luminophores with analyte-responsive groups has resulted in turn-ON probes for detection and imaging of β-galactosidase and hydrogen peroxide. The probes’ ability to image their corresponding analyte/enzyme was effectively demonstrated in vitro for β-galactosidase activity and in vivo in a mouse model of inflammation. We anticipate that our strategy for obtaining NIR luminophores will open new doors for further exploration of complex biomolecular systems using non-invasive intravital chemiluminescence imaging techniques.
Introduction
Figure 1. (A) Chemiexcitation activation mechanism of Schaap’s dioxetane (PG, protecting group). (B) Here, direct chemiluminescence amplification and red-shifted wavelength emission were obtained by a substituent effect.
Details
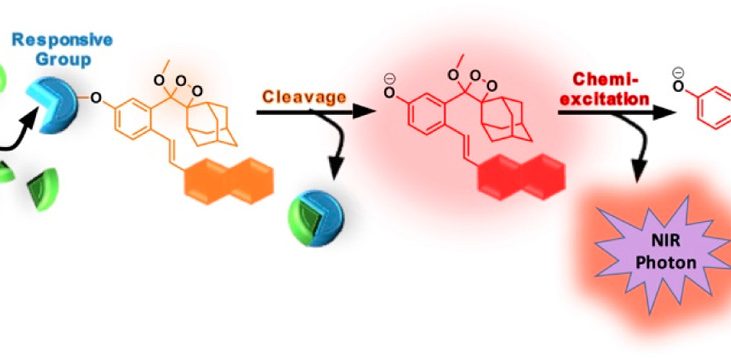
Figure 2 shows the general design and activation pathway of NIR luminophores based on Schaap’s 1,2-dioxetanes.
Figure 3 shows the molecular structure and spectral analysis of NIR luminophores 1 and 2. (Left) Chemiluminescence kinetic profile upon incubation of luminophores 1and 2 [1 μM] in PBS, pH 7.4, 10% fetal bovine serum (FBS), at 37 °C. (Right) Chemiluminescence emission spectra of luminophores 1 and 2 [50 μM] in PBS, pH 7.4, 10% FBS, at 37 °C.
Figure 4. (Top) Molecular structure of probe 1a and its activation pathway upon reaction with β-galactosidase. (Bottom) Chemiluminescence kinetic profile and total photon count of probe 1a [1 μM] in PBS, pH 7.4 (10% DMSO), in the presence or absence of 1.5 units/mL β-galactosidase.
Figure 5. (Left) Chemiluminescence imaging of probe 1a [5 μM] in HEK293 cells. (Right) Quantification of signal intensities evolving from HEK293 cells.
Figure 6. (Top) Molecular structure of probe 2a and its activation pathway upon reaction with H2O2. (Bottom) Chemiluminescence kinetic profile and total photon count of probe 2a [50 μM] in serum (5% DMSO), with and without of H2O2 [250 μM] at temperature of 37 °C.
Reference
[1]””
Name
DOI:
[2]””
Name
DOI:
[3]””
Name
DOI: